中央研究院 生物化學研究所
中央研究院 生物化學研究所
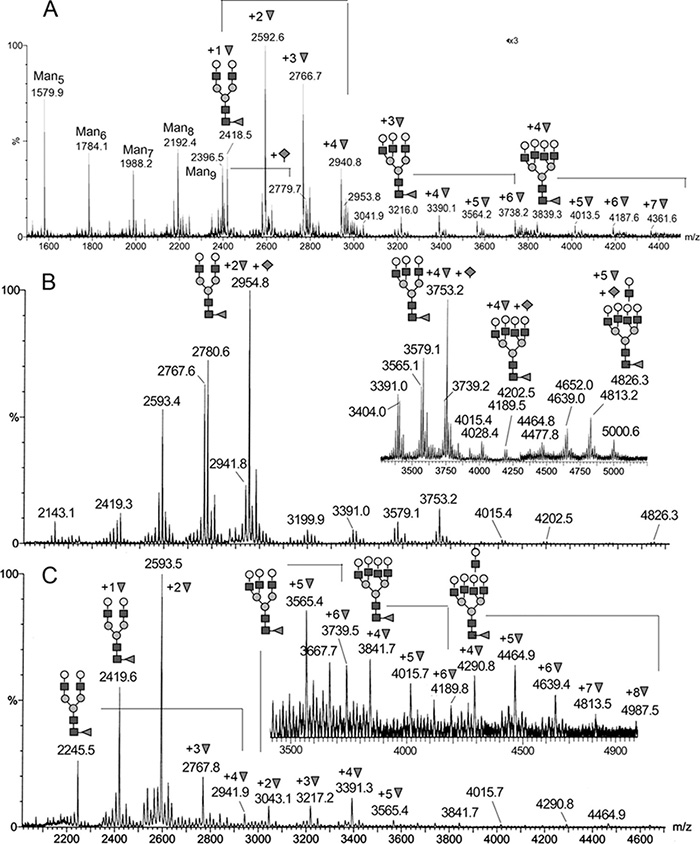
Sperm motility and maturation are known to be affected by a host of factors encountered en route in both male and female genital tracts prior to fertilization. Using a concerted proteomics and glycomics approach with advanced mass spectrometry-based glycan sequencing capability, we show in this work that 24p3, an abundant mouse uterine luminal fluid (ULF) glycoprotein also called lipocalin 2 (Lcn2), is highly fucosylated in the context of carrying multiple Lewis X and Y epitopes on complex type N-glycans at its single glycosylation site. The predominance of Lewis X/Y along with Neu5Acα2–6 sialylation was found to be a salient feature of the ULF glycome, and several other protein carriers were additionally identified including the highly abundant lactotransferrin, which is N-glycosylated at two sites, both with a similar range of highly fucosylated N-glycans. A comparative glycomics analysis of the male genital tract fluids revealed that there is a gradient of glycomic complexity from the cauda to caput regions of the epididymis, varying from high mannose to sialylated complex type N-glycans but mostly devoid of fucosylation. The seminal vesicle fluid glycome, on the other hand, carries equally abundant multimeric Lewis X structures but is distinctively lacking in additional fucosylation of the terminal galactose to give the Lewis Y epitope typifying the glycome of female ULF. One-dimensional shotgun proteomics analysis identified over 40 proteins in the latter, many of which are reported for the first time, and a majority are notably involved in immune defense and antigen processing. Further sperm binding and motility assays suggest that the Lewis X/Y epitopes do contribute to the sperm motility-enhancing activity of 24p3, whereas lactotransferrin is largely inactive in this context despite being similarly glycosylated. These findings underline the importance of glycoproteomics in delineating both the specific glycan structures and their carriers in assigning glycobiological functions.
論文網站:https://doi.org/10.1074/mcp.m800320-mcp200
作者群:Kuo CW, Chen CM, Lee YC, Chu ST*, Khoo KH*
更新時間:2009.02.01