中央研究院 生物化學研究所
中央研究院 生物化學研究所
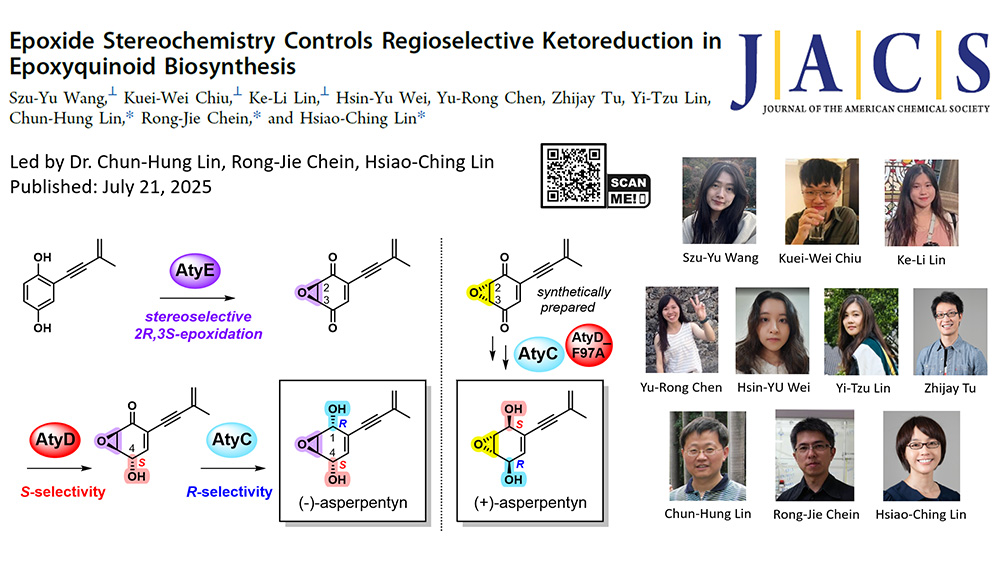
Epoxyquinoids possess multiple contiguous chiral centers and reactive functional groups, offering valuable opportunities for drug discovery and chemical biology. Despite their structural diversity, the biosynthetic pathways of these species remain poorly understood. Here, we fully elucidate the biosynthesis of (-)-asperpentyn (2) through heterologous reconstitution in Aspergillus oryzae, chemical synthesis, chiral analysis, and biochemical assays. We identify critical pathway enzymes, including the FAD-binding monooxygenase AtyG, cupin domain-containing protein AtyE, and ketoreductases AtyD and AtyC. AtyE performs oxidation and stereoselective (2R,3S)-epoxidation, converting siccayne (4) to (2R,3S)-β-epoxyquinone (6). AtyD and AtyC control S- and R-stereochemistry, respectively, for ketoreductions at C-4 and C-1 positions, with the regioselectivity of AtyD influenced by substrate epoxide configuration. Specifically, AtyD regioselectively reduces the C4-keto group on 6 but cannot further reduce the C1-keto group on (+)-harveynone (8), whereas (2S,3R>)-α-epoxyquinone (10) undergoes reduction at both C-1 and C-4 positions. Subsequent AtyC-mediated reduction installs opposite stereochemistry at C-1, yielding 2. Kinetic analyses confirm the biosynthetic sequence from 6 to 8 (AtyD) and 8 to 2 (AtyC). Mutagenesis demonstrates that residue F97 in AtyD critically determines regioselectivity, while mutations at L137A and Q246A shift the regioselectivity. Four new epoxyquinols (7, 11, 15, and 16) and four new epoxyhydroquinones (9, 12, 14, and 17) were generated, enhancing mechanistic insights into AtyD and AtyC catalyses. Notably, an in vitro one-pot reaction using AtyD_F97A and AtyC successfully converted 10 to (+)-asperpentyn (1). This comprehensive characterization of stereochemistry control in 2 biosynthesis expands enzymatic tools for generating chemical complexity among epoxyquinoid natural products.