中央研究院 生物化學研究所
中央研究院 生物化學研究所
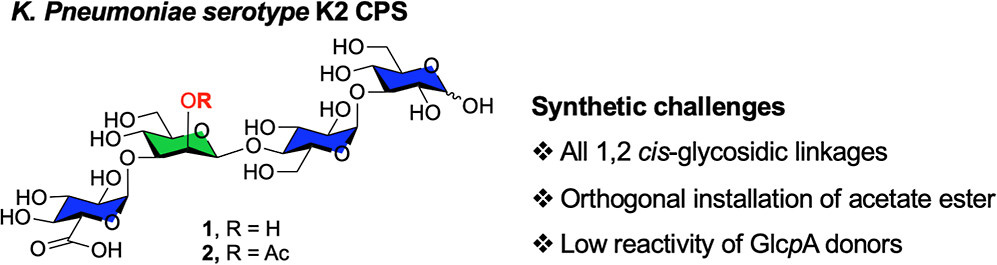
Described is the synthesis of the two tetrasaccharides (1 and 2) related to the repeating unit from the Serotype K2 capsular polysaccharide. The compounds differ by the presence or absence of an acetate ester on O-2 of the mannopyranose residue of the repeating unit. Κey challenges in the synthesis were the installation of three 1,2-cis glycosidic linkages, including a β-mannopyranoside, the selective introduction of an α-d-glucuronic acid residue, and the late-stage incorporation of an acetate ester in 2. A convergent approach employing a key [2 + 2] glycosylation was initially explored, but the key C-2 inversion needed to install the β-mannopyranoside in the resulting tetrasaccharide failed. An alternative strategy, using a sequential glycosylation approach, was successful in providing both targets. The developed route can be adapted to provide analogs containing other modifications. Such compounds are useful probes for immunological and structural biology investigations.