中央研究院 生物化學研究所
中央研究院 生物化學研究所
中央研究院 生物化學研究所

 中央研究院 生物化學研究所
中央研究院 生物化學研究所
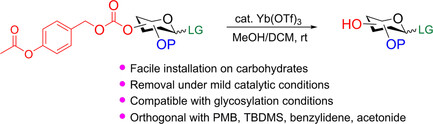
This study describes the utility of p-acetoxybenzyl carbonate (ABC) group for the protection of hydroxyl functions. This hydroxyl protecting group can be easily prepared using inexpensive commercially available 4-hydroxybenzyl alcohol as the starting material. The ABC group can be selectively removed through ytterbium(III) triflate-catalyzed transesterification at room temperature without affecting commonly used acid-cleavable protecting groups, such as tert-butyldimethylsilyl (TBDMS), p-methoxybenzyl (PMB), acetonide, and benzylidenes. Notably, the ABC protecting group is stable under glycosylation conditions in oligosaccharide synthesis.