中央研究院 生物化學研究所
中央研究院 生物化學研究所
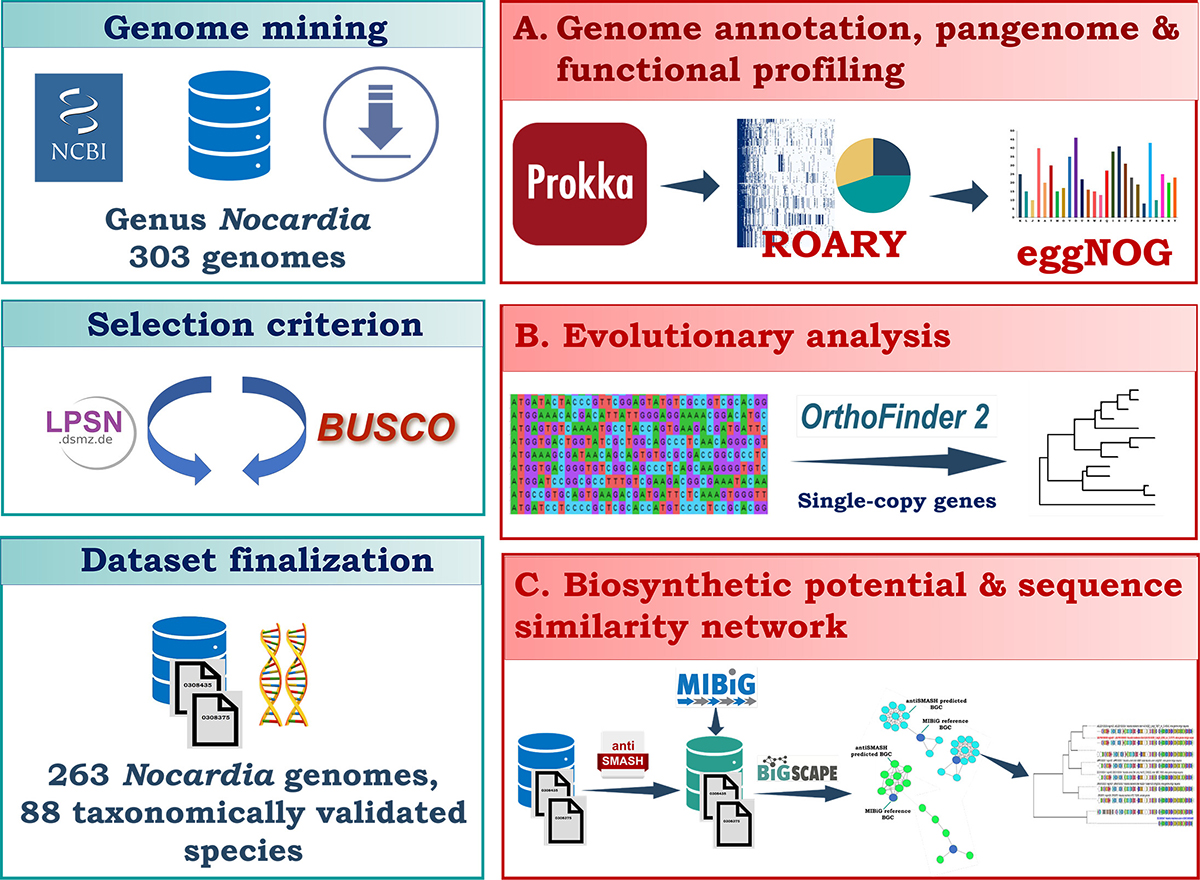
The Nocardia genus represents a largely untapped source of valuable secondary metabolites, yet its biosynthetic potential, gene content, and evolutionary history remain underexplored. By analyzing 263 genomes across 88 species, we found that Nocardia varies greatly in genome size and gene content. It exhibits an open pangenome with a small core genome (<900 genes) and high genomic fluidity (0.76), indicating high gene turnover. A large proportion (75%) of its genes are species-specific, indicating high genomic plasticity. Average nucleotide identity (ANI) analysis confirmed taxonomic relationships, with most species showing ANI values (80-85%). N. globerula showed an ANI of ~84% with Rhodococcus erythropolis, supporting its reclassification under Rhodococcus. The biosynthetic capabilities of the Nocardia genus are striking, with the presence of >8,000 biosynthetic gene clusters (BGCs), dominated by type 1 polyketide synthase, terpenes, and non-ribosomal polypeptide synthetases, establishing Nocardia as the Actinomycetota genus that has the largest biosynthetic repertoire. Around 35% of BGCs remain uncharacterized, suggesting Nocardia's high potential for novel natural product discoveries. Our study is the first to identify a prodigiosin BGC in Nocardia. Network analysis revealed complex evolutionary connections between Nocardia's gene cluster families (GCFs) and MIBiG reference BGCs, suggesting evolutionary changes, including gene gains and losses, that may have influenced the genus's BGC diversity and composition. Synteny analysis uncovered conserved and unique gene arrangements across Nocardia and related genera, mostly with core genes conserved in Actinomycetota. Our study addressed unmet clinical and biotechnological challenges while revealing evolutionary mechanisms that shape microbial diversity and adaptability.
Importance: Understanding the genomic diversity and biosynthetic potential of microorganisms is instrumental for addressing issues in microbial evolution, natural product discovery, and host-microbe interactions. Nocardia, a bacterial genus known for its opportunistic pathogenicity, represents an underexplored group of immense genomic diversity and biosynthetic capabilities. This study employed genome mining to reveal the open pangenome of Nocardia and identified an extensive repertoire of BGCs, including novel clusters with the potential to produce therapeutically significant compounds such as prodigiosin-related compounds. By integrating genome mining, phylogenetics, and synteny analysis, this study provides insights into how genomic plasticity, species-specific genes, and evolutionary changes such as gene gains and losses that contribute to Nocardia's biosynthetic diversity and evolution. These findings contribute to advancing microbial genomics, evolution, and biotechnology by uncovering the potential of Nocardia to address challenges in infectious diseases and natural product discovery. This study exemplifies how genome mining can illuminate the ecological and clinical significance of microbial diversity.