中央研究院 生物化學研究所
中央研究院 生物化學研究所
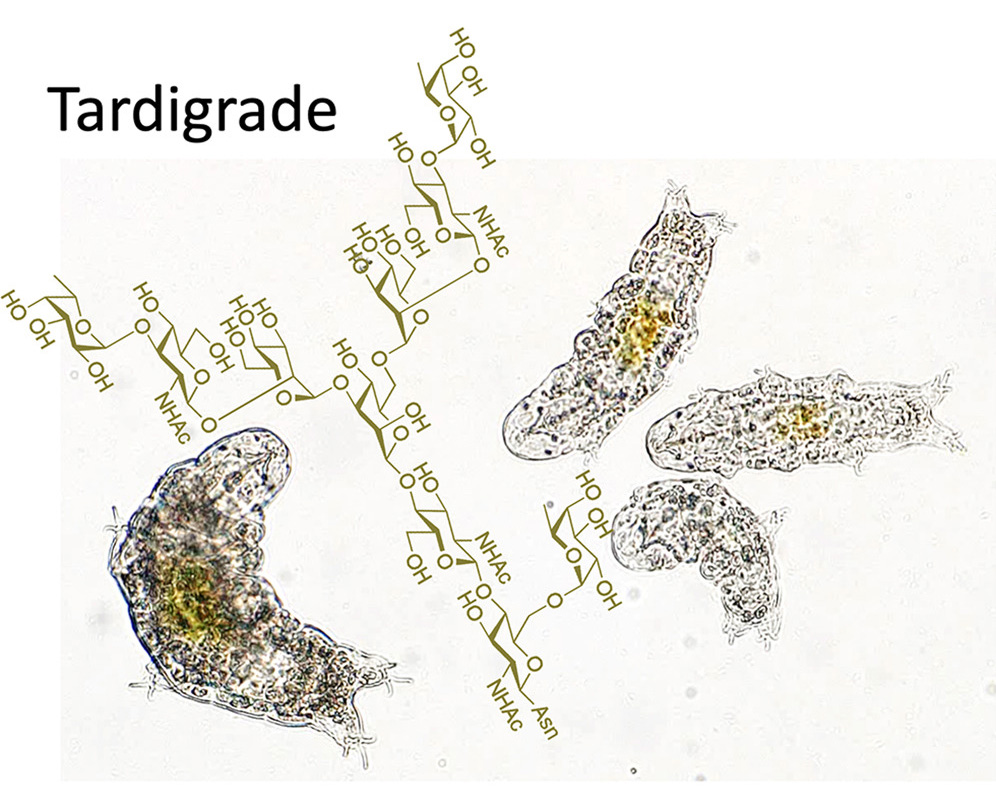
We characterized the N-glycosylation profiles of anhydrobiotic tardigrades, R. varieornatus and H. exemplaris, identifying high-mannose, paucimannose, and complex-type oligosaccharides, while hybrid-type glycans were undetectable. Notably, paucimannose-type oligosaccharides accounted for 39% of the N-glycans in R. varieornatus and 17% in H. exemplaris, with a substantial proportion of them exhibiting fucosylation of the innermost GlcNAc via an α1,6-linkage. This core fucosylation pattern, common to all animals, was observed alongside a distinctive glycosylation signature prominently observed in tardigrades: complex-type glycans lacking galactosylation but containing α1,3-fucosylated GlcNAc at non-reducing termini. This structure was more prevalent in H. exemplaris, with 22 out of 87 identified glycoproteins expressing the Fucα1,3-GlcNAc motif, including eight induced during anhydrobiosis. Key glycoproteins such as Cu/Zn-superoxide dismutase and papilin, implicated in oxidative stress protection and extracellular matrix remodeling, were among those modified. Comparative analyses reveal that non-reducing terminal α1,3-fucosylation in tardigrades is distinct from the mammalian Lewis X antigen and similar structures found in invertebrates, suggesting a unique substrate specificity of fucosyltransferases in these species. Genomic analysis identified homologs of Fut9 and FucTC, indicating potential candidates responsible for this glycosylation pattern. Our findings provide new insights into the molecular mechanisms of glycosylation in tardigrades and its relevance to their extreme stress tolerance.