中央研究院 生物化學研究所
中央研究院 生物化學研究所
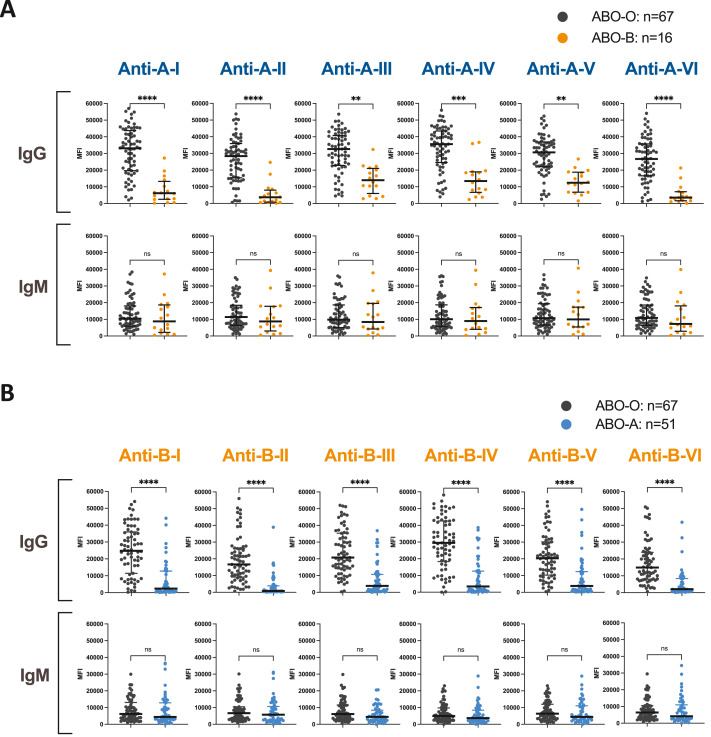
Kidney transplantation from ABO-A2 donors into ABO-O and ABO-B recipients can alleviate inequitable transplant access created by ABO demographics. ABO-A2-incompatible (ABO-A2i) eligibility is determined by anti-A hemagglutination titers. However, titers do not distinguish antibodies specific for A-II glycans, the sole A-antigen subtype in vascular endothelium, from other anti-A antibodies. We examined whether reliance on anti-A titers unnecessarily limited ABO-A2i transplants for candidates with low anti-A-II levels. We created a single-antigen bead immunoassay for ABO antibodies, confirmed the specificity and reproducibility, and demonstrated the ability to detect anti-A and anti-B glycan subtype-specific antibodies in healthy control sera. We then measured subtype-specific anti-A antibodies in original sera from ABO-B and ABO-O candidates who had been previously evaluated for ABO-A2i eligibility. Anti-A-II levels in candidates who had been deemed ineligible (anti-A titers >4) were compared to eligible candidates (anti-A titers ≤4) who had subsequently received ABO-A2i kidneys. Of 141 candidates, 75 (53%) were ineligible; 66 (47%) were eligible and received ABO-A2 kidneys. Retesting original sera, 55% (41/75) of ineligible candidates had anti-A-II levels comparable to eligible candidates. Anti-A titers did not reflect anti-A-II levels. Our ABO antibody assay reproducibly measures graft-specific anti-A-II antibodies, providing information beyond anti-A titers that may increase transplant access for ABO-B and ABO-O candidates.