中央研究院 生物化學研究所
中央研究院 生物化學研究所
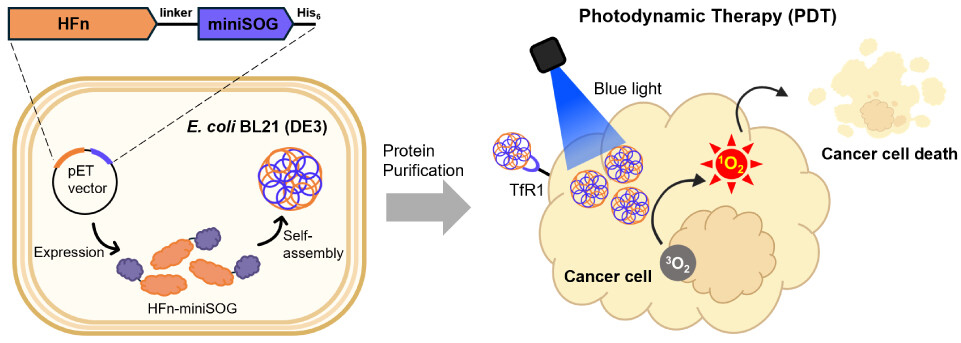
To avoid off-target effects, targeted cancer therapy offers a feasible alternative to traditional cancer therapies such as chemotherapy and radiotherapy. The human ferritin receptor (transferrin receptor, TfR1) is greatly overexpressed in several cancer types, including liver cancer. Therefore, human ferritin (HFn) has been used in drug encapsulation for targeted therapy. However, the drug encapsulation method is time-consuming and not applicable to all conditions. In this study, we effectively designed HFn fused with a photosensitizing protein called mini-singlet oxygen generator (miniSOG) to create HFn-miniSOG for targeted photodynamic therapy (PDT) applications. The fusion protein HFn-miniSOG self-assembled to form nanoparticles with an average size of 22.4 ± 1.3 nm and generated singlet oxygen (1O2) when activated by blue-light irradiation with ΦΔ = 0.30. To demonstrate its targeted PDT capability, phototoxicity was assessed in HepG2 and HeLa cells with varying TfR1 expression levels. The viability of HepG2 cells was reduced by 63% after light irradiation, compared to 34% in HeLa cells, because HepG2 cells exhibit greater levels of TfR1. As a result, our study provides a straightforward approach for creating ALL-IN-ONE protein nanoparticles for targeted PDT applications.