中央研究院 生物化學研究所
中央研究院 生物化學研究所
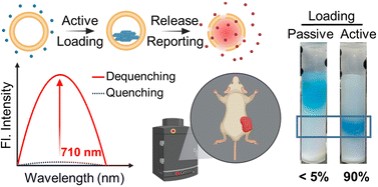
Visualizing liposome release profiles in small animals is important for evaluating the pharmacokinetic influence of vesicles. Encapsulating near-infrared (NIR) fluorescent dyes to visualize and report liposomal cargo release in vivo, which necessitates high encapsulation with deep self-quenching, is highly desirable in advanced (such as targeting or trigger-release) liposome development. However, passive loading of NIR dyes usually yields low encapsulation efficiencies (1–5%), causing significant wastage and cost-ineffectiveness while using expensive NIR fluorescent dyes. It would be highly beneficial if an active loading method, which typically has an encapsulation efficiency of nearly 100%, is developed. This research describes an active loading approach for two cyanine 5.5 (Cy5.5) derivatives. We discovered that using ammonium sucrose octasulfate (ASO) as a trapping agent allows for nearly 100% encapsulation for both Cy5.5 dyes, accompanied by the formation of nanoprecipitates inside the liposome, as evidenced by cryogenic electron microscopy. Fluorescence spectroscopy confirmed deep fluorescence self-quenching after active loading and a 60–100-fold fluorescence enhancement upon full content release via liposome rupture. Cellular uptake experiments showed that the fluorescence of Cy5.5-loaded liposomes recovered and plateaued after 9 hours of incubation with cells. In vivo fluorescence imaging (IVIS) demonstrated the same fluorescence activation in tumor-bearing mice intratumorally injected with the liposome. We believe that the developed active loading method will enable Cy5.5-loaded liposomes to be a deep tissue-compatible and cost-effective NIR fluorescence release-reporting platform.