中央研究院 生物化學研究所
中央研究院 生物化學研究所
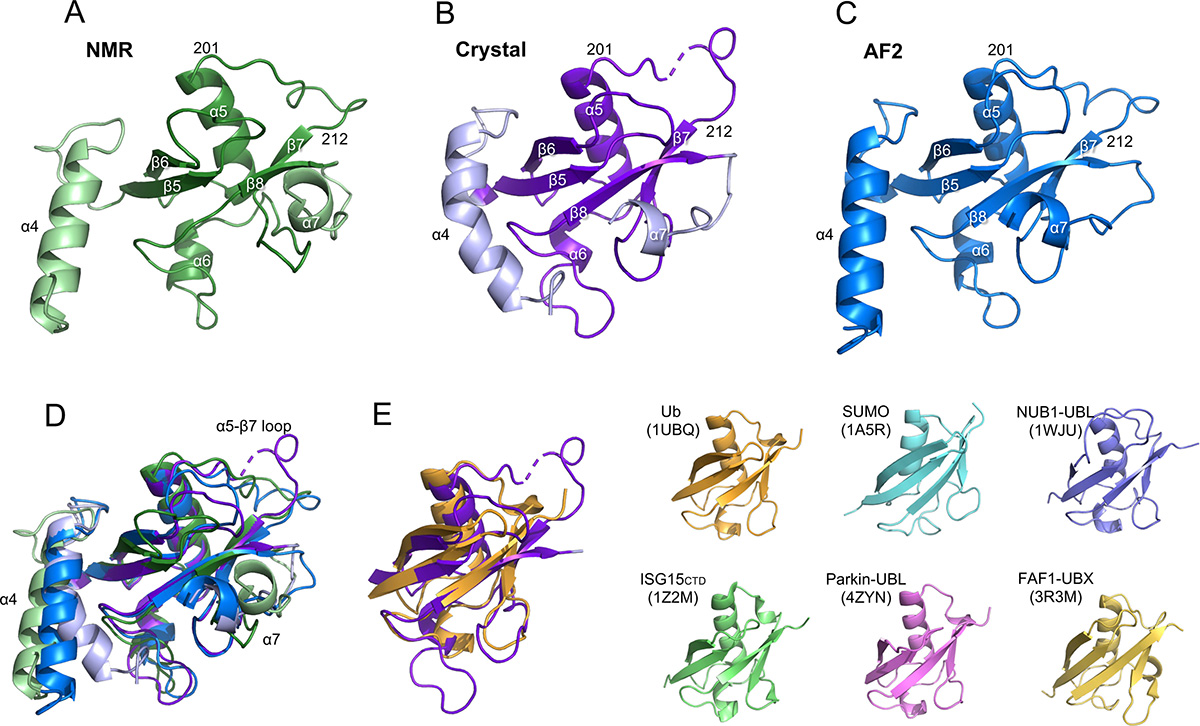
Arsenite-induced stress granule (SG) formation can be cleared by the ubiquitin-proteasome system aided by the ATP-dependent unfoldase p97. ZFAND1 participates in this pathway by recruiting p97 to trigger SG clearance. ZFAND1 contains two An1-type zinc finger domains (ZF1 and ZF2), followed by a ubiquitin-like domain (UBL); but their structures are not experimentally determined. To shed light on the structural basis of the ZFAND1-p97 interaction, we determined the atomic structures of the individual domains of ZFAND1 by solution-state nuclear magnetic resonance (NMR) spectroscopy and X-ray crystallography. We further characterized the interaction between ZFAND1 and p97 by methyl NMR spectroscopy and cryo-electron microscopy (cryo-EM). 15N spin relaxation dynamics analysis indicated independent domain motions for ZF1, ZF2, and UBL. The crystal structure and NMR structure of UBL showed a conserved β-grasp fold homologus to ubiquitin and other UBLs. Nevertheless, the UBL of ZFAND1 contains an additional N-terminal helix that adopts different conformations in the crystalline and solution states. ZFAND1 uses the C-terminal UBL to bind to p97, evidenced by the pronounced line-broadening of the UBL domain during the p97 titration monitored by methyl NMR spectroscopy. ZFAND1 binding induces pronounced conformational heterogeneity in the N-terminal domain (NTD) of p97, leading to a partial loss of the cryo-EM density of the NTD of p97. In conclusion, this work paved the way for a better understanding of the interplay between p97 and ZFAND1 in the context of SG clearance.