中央研究院 生物化學研究所
中央研究院 生物化學研究所
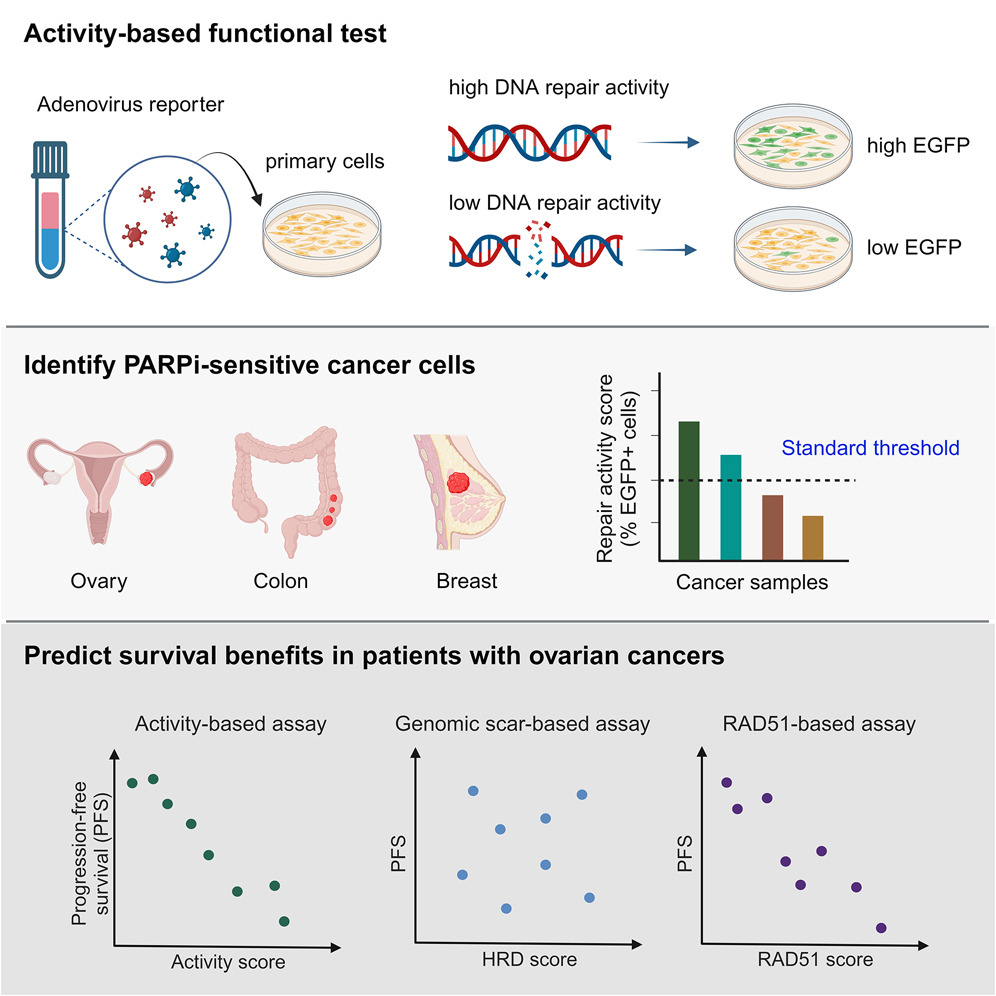
Homologous recombination (HR)-mediated DNA repair is a prerequisite for maintaining genome stability. Cancer cells displaying HR deficiency (HRD) are selectively eliminated by poly(ADP-ribose) polymerase inhibitors (PARPis). To date, sequencing of HR-associated genes and analyzing genome instability have been used as clinical predictions for PARPi therapy. However, these genetic tests cannot reflect dynamic changes in the HR status. Here, we have developed a virus- and activity-based functional assay to quantify real-time HR activity directly. Instead of focusing on a few HR-associated genes, our functional assay detects endpoint HR activity and establishes an activity threshold for identifying HRD across cancer types, validated by PARPi sensitivity and BRCA status. Notably, this fluorescence-based assay can be applied to primary ovarian cancer cells from patients to reflect their level of HRD, which is associated with survival benefits. Thus, our work provides a functional test to predict the response of primary cancer cells to PARPis.