中央研究院 生物化學研究所
中央研究院 生物化學研究所
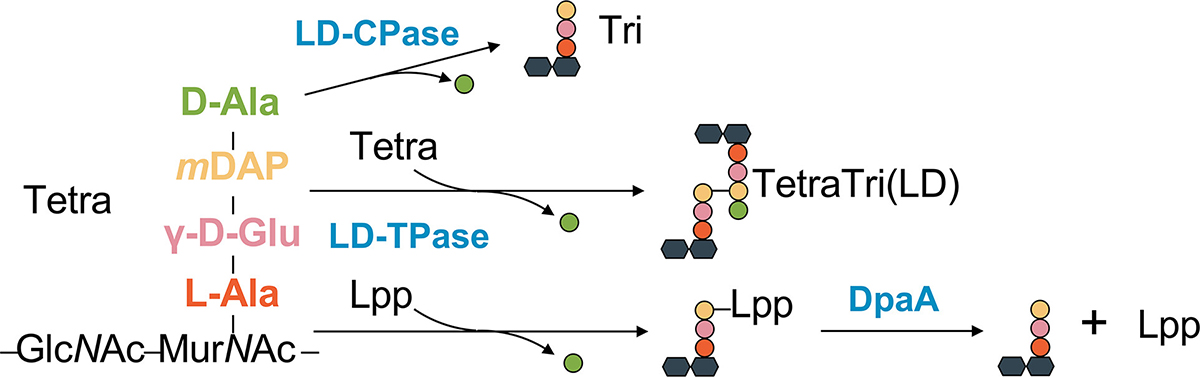
The peptidoglycan layer is a defining characteristic of bacterial cells, providing them with structural support and osmotic protection. In Escherichia coli, this layer is linked to the outer membrane via the abundant membrane-anchored protein Lpp, known as Braun’s lipoprotein, with LD-transpeptidases LdtA, LdtB, and LdtC catalyzing the attachment. However, one distinctive member of the YkuD-type transpeptidase family, LdtF (recently renamed DpaA), carries out the opposite reaction of detaching Lpp from the peptidoglycan layer. In this study, we report the crystal structure of DpaA, which reveals the enzyme’s ability to cleave, rather than form, the Lpp-peptidoglycan linkage. Assays with purified peptidoglycan-Lpp as the substrate and chemically synthesized compounds suggest that DpaA’s shallow L-shaped active site can only accommodate and cleave the peptidoglycan-Lpp cross-link with a constrained conformation. This study provides insights into how homologous Ldt enzymes can perform opposing chemical reactions.