中央研究院 生物化學研究所
中央研究院 生物化學研究所
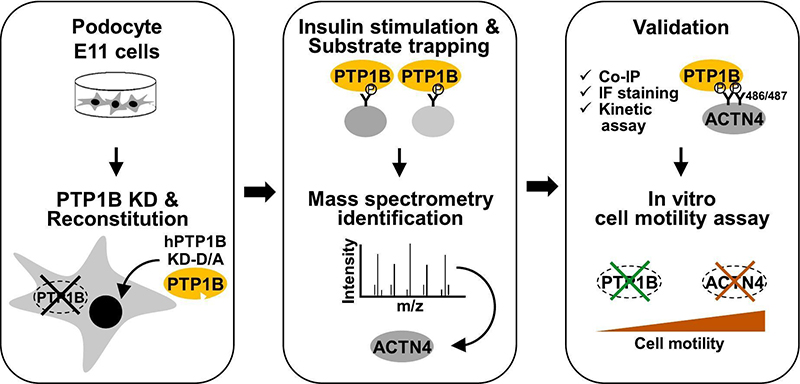
Glomerular podocytes are instrumental for the barrier function of the kidney, and podocyte injury contributes to proteinuria and the deterioration of renal function. Protein tyrosine phosphatase 1B (PTP1B) is an established metabolic regulator, and the inactivation of this phosphatase mitigates podocyte injury. However, there is a paucity of data regarding the substrates that mediate PTP1B actions in podocytes. This study aims to uncover novel substrates of PTP1B in podocytes and validate a leading candidate. To this end, using substrate-trapping and mass spectroscopy, we identified putative substrates of this phosphatase and investigated the actin cross-linking cytoskeletal protein alpha-actinin4. PTP1B and alpha-actinin4 co-localized in murine and human glomeruli and transiently transfected E11 podocyte cells. Additionally, podocyte PTP1B deficiency in vivo and culture was associated with elevated tyrosine phosphorylation of alpha-actinin4. Conversely, reconstitution of the knockdown cells with PTP1B attenuated alpha-actinin4 tyrosine phosphorylation. We demonstrated co-association between alpha-actinin4 and the PTP1B substrate-trapping mutant, which was enhanced upon insulin stimulation and disrupted by vanadate, consistent with an enzyme-substrate interaction. Moreover, we identified alpha-actinin4 tandem tyrosine residues 486/487 as mediators of its interaction with PTP1B. Furthermore, knockdown studies in E11 cells suggest that PTP1B and alpha-actinin4 are modulators of podocyte motility. These observations indicate that PTP1B and alpha-actinin4 are likely interacting partners in a signaling node that modulates podocyte function. Targeting PTP1B and plausibly this one of its substrates may represent a new therapeutic approach for podocyte injury that warrants additional investigation.