中央研究院 生物化學研究所
中央研究院 生物化學研究所
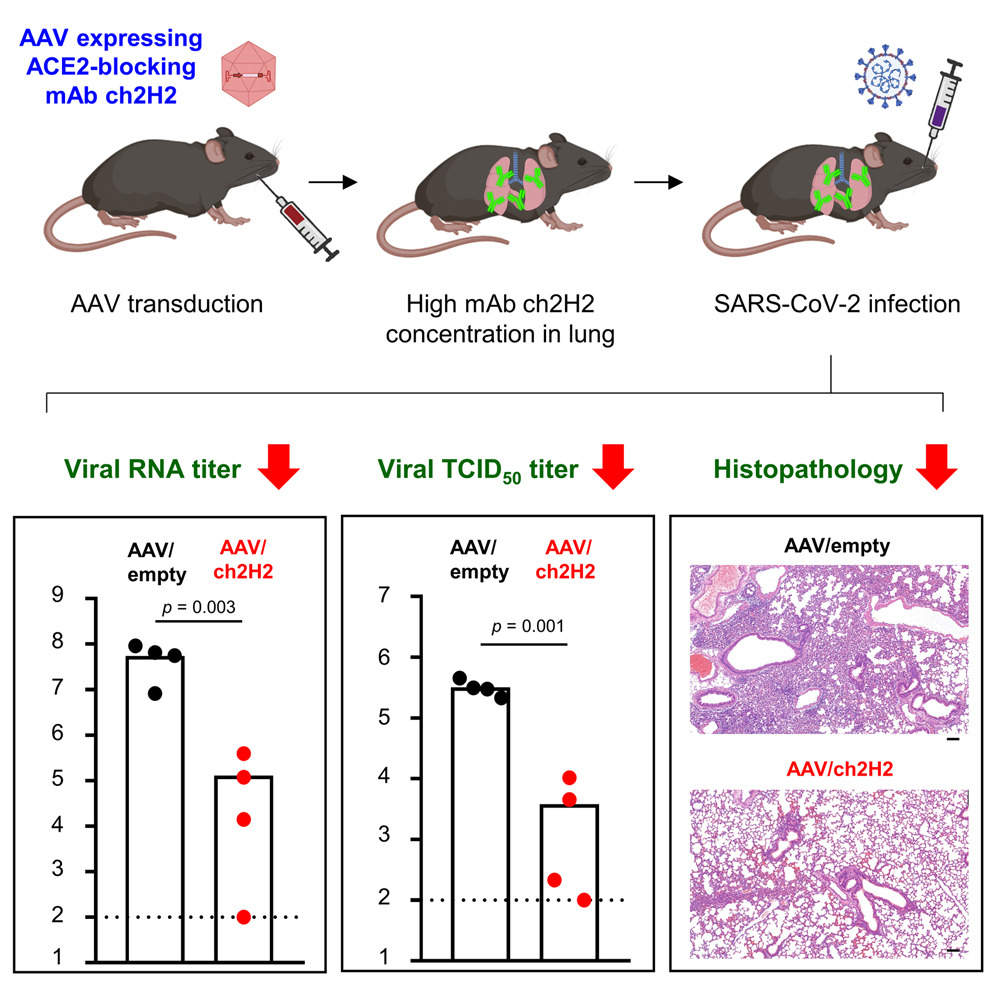
The ongoing evolution of SARS-CoV-2, resulting in the emergence of new variants that are resistant to existing vaccines and therapeutic antibodies, has raised the need for novel strategies to combat the persistent global COVID-19 epidemic. In this study, a monoclonal anti-human angiotensin-converting enzyme 2 (hACE2) antibody, ch2H2, was isolated and humanized to block the viral receptor-binding domain (RBD) binding to hACE2, the major entry receptor of SARS-CoV-2. This antibody targets the RBD binding site on the N-terminus of hACE2 and has a high binding affinity to outcompete the RBD. In vitro, ch2H2 antibody showed potent inhibitory activity against multiple SARS-CoV-2 variants, including the most antigenically drifted and immune-evading variant Omicron. In vivo, adeno-associated virus (AAV)-mediated delivery enabled a sustained expression of mAb ch2H2, generating a high concentration of antibodies in mice. A single administration of AAV-delivered mAb ch2H2 significantly reduced viral RNA load and infectious virions and mitigated pulmonary pathological changes in mice challenged with SARS-CoV-2 Omicron BA.5 subvariant. Collectively, the results suggest that AAV-delivered hACE2-blocking antibody provides a promising approach for developing broad-spectrum antivirals against SARS-CoV-2 and potentially other hACE2-dependent pathogens that may emerge in the future.