中央研究院 生物化學研究所
中央研究院 生物化學研究所
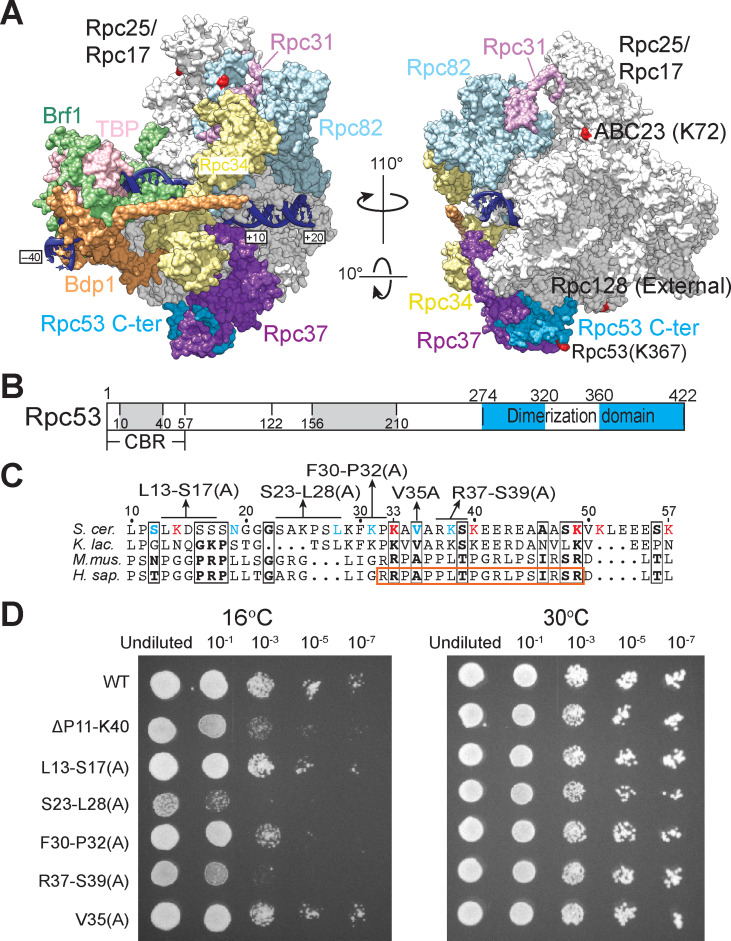
The TFIIF-like Rpc53/Rpc37 heterodimer of RNA polymerase (pol) III is involved in various stages of transcription. The C-terminal region of Rpc53 dimerizes with Rpc37 to anchor on the lobe domain of the pol III cleft. However, structural and functional features of the Rpc53 N-terminal region had not been characterized previously. Here, we conducted site-directed alanine replacement mutagenesis on the Rpc53 N-terminus, generating yeast strains that exhibited a cold-sensitive growth defect and severely compromised pol III transcriptional activity. Circular dichroism and NMR spectroscopy revealed a highly disordered 57-amino acid polypeptide in the Rpc53 N-terminus. This polypeptide is a versatile protein-binding module displaying nanomolar-level binding affinities for Rpc37 and the Tfc4 subunit of the transcription initiation factor TFIIIC. Accordingly, we denote this Rpc53 N-terminus polypeptide as the TFIIIC-binding region or CBR. Alanine replacements in the CBR significantly reduced its binding affinity for Tfc4, highlighting its functional importance to cell growth and transcription in vitro. Our study reveals the functional basis for Rpc53's CBR in assembly of the pol III transcription initiation complex.