中央研究院 生物化學研究所
中央研究院 生物化學研究所
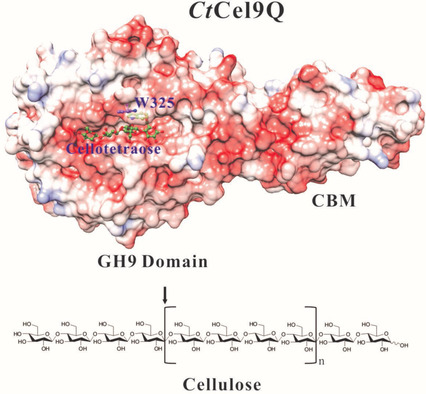
Endoglucanase CtCel9Q is one of the enzyme components of the cellulosome, which is an active cellulase system in the thermophile Clostridium thermocellum. The precursor form of CtCel9Q comprises a signal peptide, a glycoside hydrolase family 9 catalytic domain, a type 3c carbohydrate-binding module (CBM), and a type I dockerin domain. Here, we report the crystal structures of C-terminally truncated CtCel9Q (CtCel9QΔc) complexed with Tris, Tris+cellobiose, cellobiose+cellotriose, cellotriose, and cellotetraose at resolutions of 1.50, 1.70, 2.05, 2.05 and 1.75 Å, respectively. CtCel9QΔc forms a V-shaped homodimer through residues Lys529–Glu542 on the type 3c CBM, which pairs two β-strands (β4 and β5 of the CBM). In addition, a disulfide bond was formed between the two Cys535 residues of the protein monomers in the asymmetric unit. The structures allow the identification of four minus (−) subsites and two plus (+) subsites; this is important for further understanding the structural basis of cellulose binding and hydrolysis. In the oligosaccharide-free and cellobiose-bound CtCel9QΔc structures, a Tris molecule was found to be bound to three catalytic residues of CtCel9Q and occupied subsite −1 of the CtCel9Q active-site cleft. Moreover, the enzyme activity assay in the presence of 100 mm Tris showed that the Tris almost completely suppressed CtCel9Q hydrolase activity.