中央研究院 生物化學研究所
中央研究院 生物化學研究所
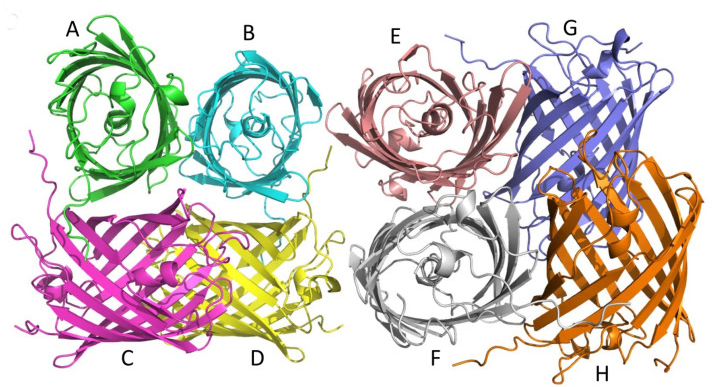
Chromoproteins are a good source of engineered biological tools. We previously reported the development of a blue fluorescent protein, termed shBFP, which was derived from a purple chromoprotein shCP found in the sea anemone Stichodacyla haddoni. shBFP contains a Leu63-Leu64-Gly65 tri-peptide chromophore, and shows maximum excitation and emission wavelengths at 401 nm and 458 nm, along with a high quantum yield. How this chromophore endows shBFP with the unique fluorescence property in the absence of a hydroxyphenyl ring remained unclear. Here, we present the crystal structures of shCP and shBFP at 1.9- and 2.05-Å resolution, respectively. Both proteins crystallized as similar tetramers, but they are more likely to function as dimers in solution. The chromophore in shCP shows a trans-conformation and its non-planarity is similar to most other homologues. The shBFP chromophore also contains an imidazolidone moiety in its structure, but there are a smaller number of conjugated double bonds compared to shCP. Consequently, the chromophore may prefer absorbing shorter wavelength lights in the UV region, followed by the emission of blue fluorescence. These observations provide new insights into the molecular basis that correlates chromophore conformation with light absorption and fluorescence emission for the development of improved biomarkers.