中央研究院 生物化學研究所
中央研究院 生物化學研究所
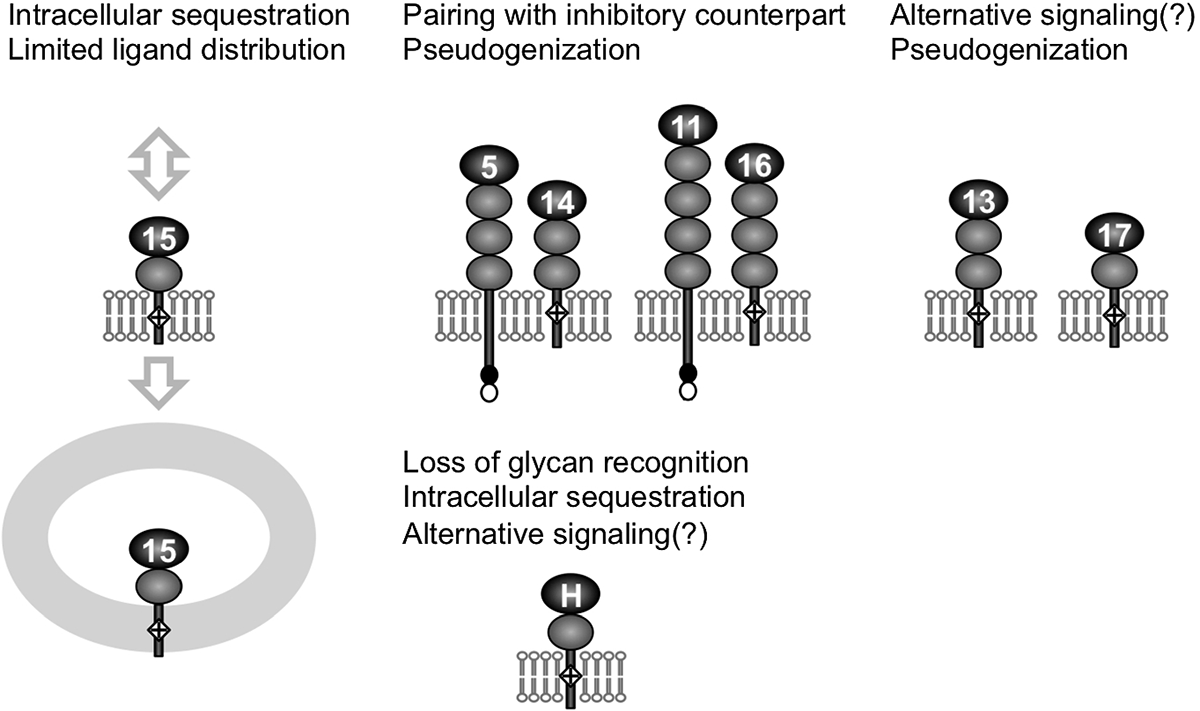
Siglecs are a family of transmembrane receptor-like glycan-recognition proteins expressed primarily on leukocytes. Majority of Siglecs have an intracellular sequence motif called immunoreceptor tyrosine-based inhibitory motif (ITIM) and associate with Src homology region 2 domain-containing tyrosine phosphatase-1 (SHP-1), and negatively regulate tyrosine phosphorylation-mediated intracellular signaling events. On the other hand, some Siglecs have a positively charged amino acid residue in the transmembrane domain and associate with DNAX activation protein of 12 kDa (DAP12), which in turn recruits spleen tyrosine kinase (Syk). These DAP12-associated Siglecs play diverse functions. For example, Siglec-15 is conserved throughout vertebrate evolution and plays a role in bone homeostasis by regulating osteoclast development and function. Human Siglec-14 and -16 have inhibitory counterparts (Siglec-5 and -11, respectively), which show extremely high sequence similarity with them at the extracellular domain but interact with SHP-1. The DAP12-associated Siglec in such "paired receptor" configuration counteracts the pathogens that exploit the inhibitory counterpart. Polymorphisms (mutations) that render DAP12-associated inactive Siglecs are found in humans, and some of these appear to be associated with sensitivity or resistance of human hosts to bacterially induced conditions. Studies of mouse Siglec-H have revealed complex and intriguing functions it plays in regulating adaptive immunity. Many questions remain unanswered, and further molecular and genetic studies of DAP12-associated Siglecs will yield valuable insights with translational relevance.