中央研究院 生物化學研究所
中央研究院 生物化學研究所
Background: Aberrant fucosylation plays a critical role in lung cancer progression. Nevertheless, the key fucosyltransferase with prognostic significance in lung cancer patients, the enzyme's intracellular targets, and complex molecular mechanisms underlying lung cancer metastasis remain incompletely understood.
Methods: We performed a large-scale transcriptome-clinical correlation to identify major fucosyltransferases with significant prognostic values. Invasion, migration, cell adhesion assays were performed using lung cancer cells subject to genetic manipulation of FUT4 levels. Genome-wide RNA-seq and immunoprecipitation-mass spectrometry were used to characterize major cellular processes driven by FUT4, as well as profiling its intracellular protein targets. We also performed lung homing and metastasis assays in mouse xenograft models to determine in vivo phenotypes of high FUT4-expressing cancer cells.
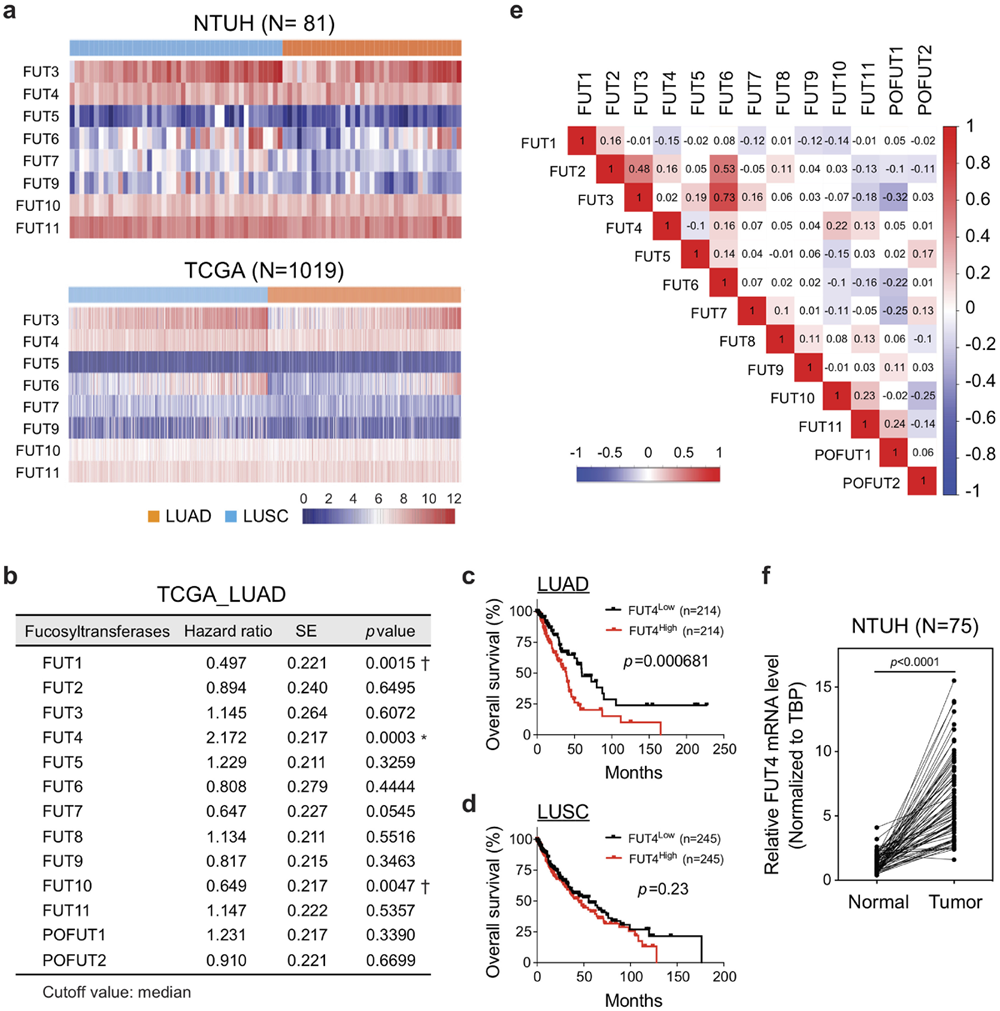
Findings: We show that FUT4 is associated with poor overall survival in lung adenocarcinoma patients. High FUT4 expression promotes lung cancer invasion, migration, epithelial-to-mesenchymal transition, and cell adhesion. FUT4-mediated aberrant fucosylation markedly activates multiple cellular processes, including membrane trafficking, cell cycle, and major oncogenic signaling pathways. The effects are independent of receptor tyrosine kinase mutations. Notably, genetic depletion of FUT4 or targeting FUT4-driven pathways diminishes lung colonization and distant metastases of lung cancer cells in mouse xenograft models.
Interpretation: We propose that FUT4 can be a prognostic predictor and therapeutic target in lung cancer metastasis. Our data provide a scientific basis for a potential therapeutic strategy using targeted therapy in a subset of patients with high FUT4-expressing tumors with no targetable mutations.
Keywords: Cancer metastasis; Fucosyltransferase; Glycoproteomics; Lung cancer; Terminal fucosylation.