中央研究院 生物化學研究所
中央研究院 生物化學研究所
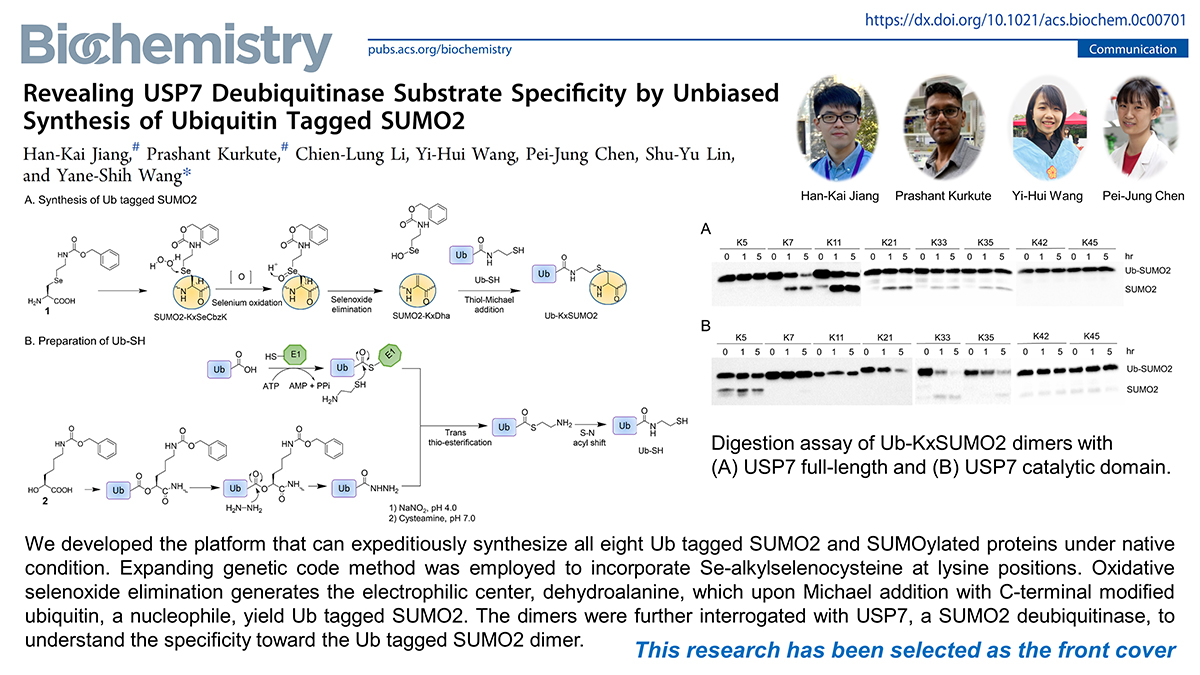
Ubiquitination and SUMOylation of protein are crucial for various biological responses. The recent unraveling of cross-talk between SUMO and ubiquitin (Ub) has shown the pressing needs to develop the platform for the synthesis of Ub tagged SUMO2 dimers to decipher its biological functions. Still, the platforms for facile synthesis of dimers under native condition are less explored and remain major challenges. Here, we have developed the platform that can expeditiously synthesize all eight Ub tagged SUMO2 and SUMOylated proteins under native condition. Expanding genetic code (EGC) method was employed to incorporate Se-alkylselenocysteine at lysine positions. Oxidative selenoxide elimination generates the electrophilic center, dehydroalanine, which upon Michael addition with C-terminal modified ubiquitin, a nucleophile, yield Ub tagged SUMO2. The dimers were further interrogated with USP7, a SUMO2 deubiquitinase, which is involved in DNA repair, to understand specificity toward the Ub tagged SUMO2 dimer. Our results have shown that the C-terminal domain of USP7 is crucial for USP7 efficiency and selectivity for the Ub tagged SUMO2 dimer.