中央研究院 生物化學研究所
中央研究院 生物化學研究所
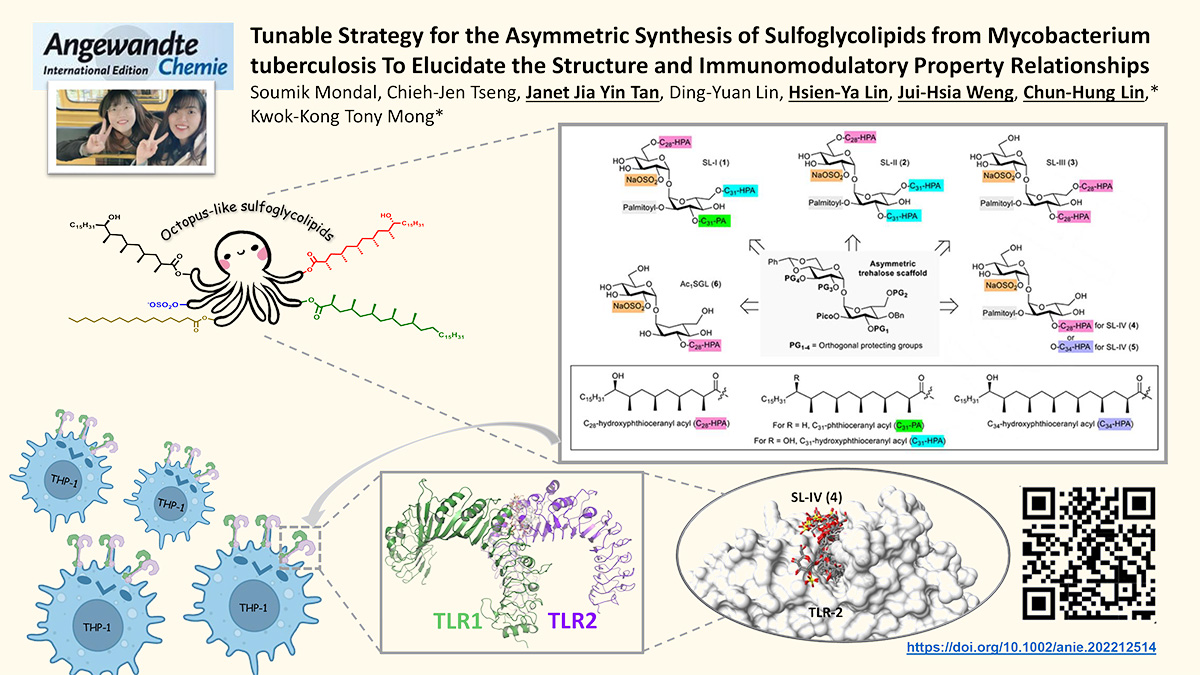
We developed a versatile asymmetric strategy to synthesize different classes of sulfoglycolipids (SGLs) from Mycobacterium tuberculosis , namely SL-I ( 1 ), SL-II ( 2 ), SL-III ( 3 ), SL-IVs ( 4 ), ( 5 ), and monoacyl Ac 1 SGL ( 6 ). The strategy featured the use of asymmetrically-protected trehaloses, which were acquired from glycosylation of TMS α -glucosyl acceptors with benzylidene protected thioglucosyl donors. The positions of the protecting groups at the donors and acceptors can be fine-tuned to obtain different protecting group patterns, which are crucial for regioselective acylation and sulfation. In addition, a chemoenzymatic strategy was established to prepare the polymethylated fatty acid building blocks. The strategy employs (i) inexpensive lipase as a desymmetrization agent in preparation of starting substrate and (ii) readily-available chiral oxazolidinone as a chiral-controlling agent in construction of the polymethylated fatty acids. Subsequent investigation on immunomodulatory property of each class of SGLs showed how the structures of SGLs impact the host innate immunity response.