中央研究院 生物化學研究所
中央研究院 生物化學研究所
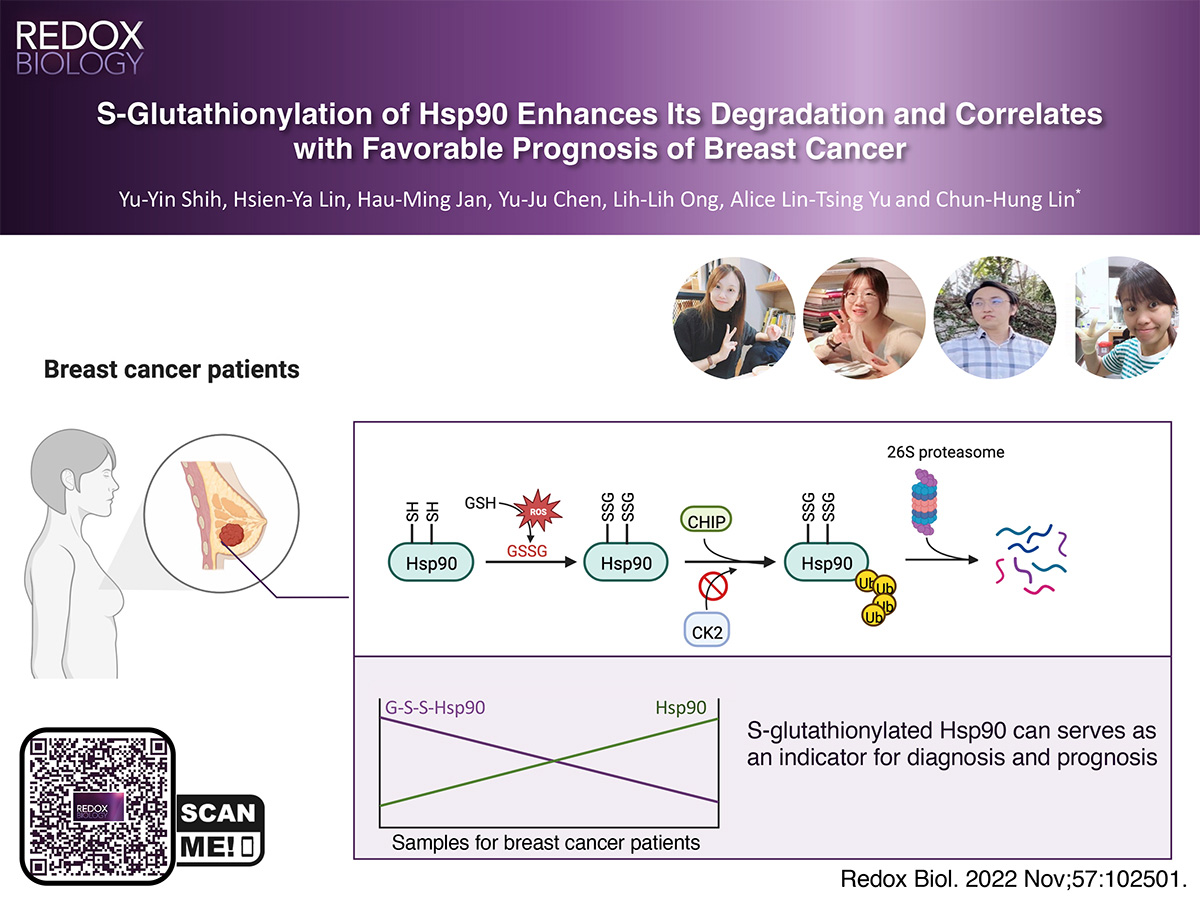
Heat shock protein 90 (Hsp90) is a ubiquitous chaperone to interact with numerous proteins to regulate multiple cellular processes, especially during cell proliferation and cell cycle progression. Hsp90 exists in a high level in tumor cells and tissues, and thus serves as a prognostic biomarker or therapeutic target in cancers. We herein report that Hsp90 is subjected to S-glutathionylation, a redox-dependent modification to form a disulfide bond between the tripeptide glutathione and cysteine residues of proteins, primarily at C366 and C412 in the presence of reactive oxygen species. The modification led to the loss of the ATPase activity. The level of Hsp90 was obviously reduced by S-glutathionylation, owing to C-terminus of Hsc70-interacting protein (CHIP)-mediated ubiquitin proteasome system. S-glutathionylation of Hsp90 was found to crosstalk with its C-terminal phosphorylation of Hsp90 that impedes the binding of Hsp90 with CHIP, demonstrating the importance of chaperone code in modulating Hsp90 function. Further biophysical analyses indicated that S-glutathionylation caused structural change of Hsp90, underlying the aforementioned functional regulation. Moreover, in accordance with the analysis of 64 samples collected from patients of breast cancer, the expression level of Hsp90 inversely correlated with the glutathionylated status of Hsp90. The ratio of total expression to glutathionylated status of Hsp90 was coherent to expression of biomarkers in breast cancer sample, potentiating the prognostic value in the cancer treatment.