中央研究院 生物化學研究所
中央研究院 生物化學研究所
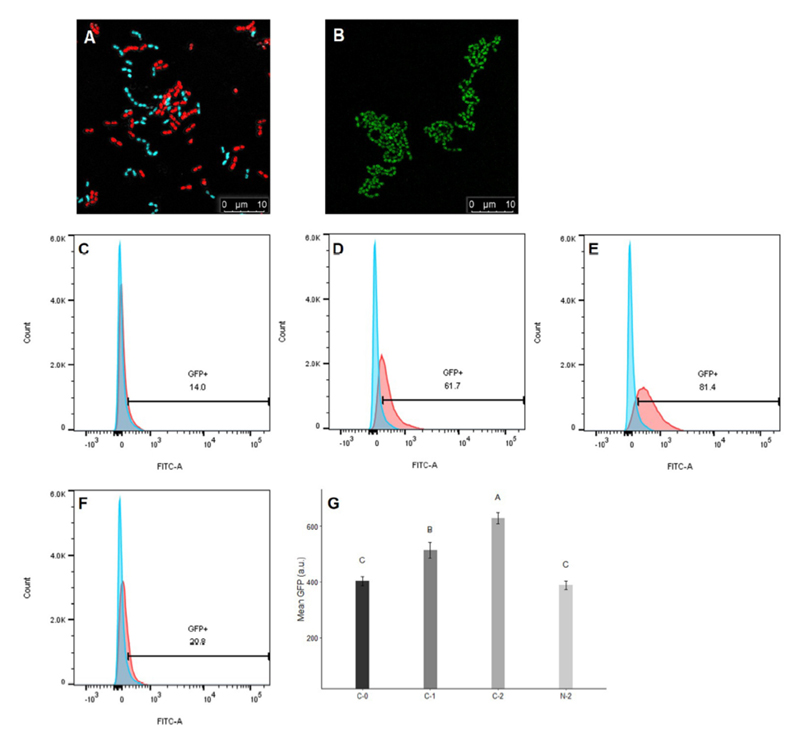
Enterococcus faecalis, a member of the commensal flora in the human gastrointestinal tract, has become a threatening nosocomial pathogen because it has developed resistance to many known antibiotics. More concerningly, resistance gene-carrying E. faecalis cells may transfer antibiotic resistance to resistance-free E. faecalis cells through their unique quorum sensing-mediated plasmid transfer system. Therefore, we investigated the role of probiotic bacteria in the transfer frequency of the antibiotic resistance plasmid pCF10 in E. faecalis populations to mitigate the spread of antibiotic resistance. Bacillus subtilis subsp. natto is a probiotic strain isolated from Japanese fermented soybean foods, and its culture fluid potently inhibited pCF10 transfer by suppressing peptide pheromone activity from chromosomally encoded CF10 (cCF10) without inhibiting E. faecalis growth. The inhibitory effect was attributed to at least one 30- to 50-kDa extracellular protease present in B. subtilis subsp. natto. Nattokinase of B. subtilis subsp. natto was involved in the inhibition of pCF10 transfer and cleaved cCF10 (LVTLVFV) into LVTL plus VFV fragments. Moreover, the cleavage product LVTL (L peptide) interfered with the conjugative transfer of pCF10. In addition to cCF10, faecalis-cAM373 and gordonii-cAM373, which are mating inducers of vancomycin-resistant E. faecalis, were also cleaved by nattokinase, indicating that B. subtilis subsp. natto can likely interfere with vancomycin resistance transfer in E. faecalis. Our work shows the feasibility of applying fermentation products of B. subtilis subsp. natto and L peptide to mitigate E. faecalis antibiotic resistance transfer.
IMPORTANCE Enterococcus faecalis is considered a leading cause of hospital-acquired infections. Treatment of these infections has become a major challenge for clinicians because some E. faecalis strains are resistant to multiple clinically used antibiotics. Moreover, antibiotic resistance genes can undergo efficient intra- and interspecies transfer via E. faecalis peptide pheromone-mediated plasmid transfer systems. Therefore, this study provided the first experimental demonstration that probiotics are a feasible approach for interfering with conjugative plasmid transfer between E. faecalis strains to stop the transfer of antibiotic resistance. We found that the extracellular protease(s) of Bacillus subtilis subsp. natto cleaved peptide pheromones without affecting the growth of E. faecalis, thereby reducing the frequency of conjugative plasmid transfer. In addition, a specific cleaved pheromone fragment interfered with conjugative plasmid transfer. These findings provide a potential probiotic-based method for interfering with the transfer of antibiotic resistance between E. faecalis strains.