中央研究院 生物化學研究所
中央研究院 生物化學研究所
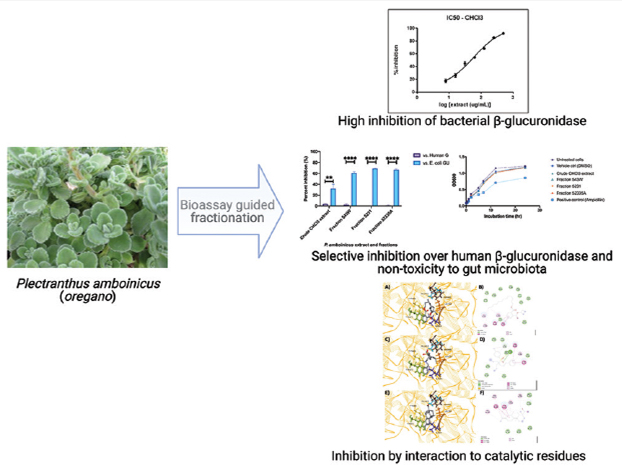
Background: Plectranthus amboinicus (Spreng.) is an herb commonly used in folk medicine and food by several Asian countries. The bioactivities of this medicinal plant have so far not been linked to a specific enzyme target. Bacterial beta-glucuronidases (β-GUS) expressed by human gut microbiota affect xenobiotic processing by reactivating toxic substances (e.g., anticancer drugs, nonsteroidal anti-inflammatory drugs, and food carcinogens) in the gut lumen. Objectives: An approach to alleviating the toxic effects of these compounds is by inhibiting bacterial β-GUS. Materials and Methods: We determined the Escherichia coli β-GUS inhibitory activity of P. amboinicus leaves using a bioassay-guided purification approach. The P. amboinicus chloroform extract was purified using normal-phase column chromatography to produce several fractions. The fractions were screened for E. coli β-GUS inhibitory activity using the 4-Methylumbelliferyl glucuronide (4-MUG) assay. Fractions with high activity were assayed further to determine toxicity against E. coli and selectivity compared to human β-GUS. Highly-active and highly-selective fractions were further characterized using gas chromatography-mass spectrometry (GC-MS) and in silico docking to identify specific compounds. Results: Assay-guided purification of the crude chloroform extract with β-GUS inhibitory activity (IC50 = 57.8 μg/mL) yielded four fractions with high activity: Fraction-543W (IC50 = 16.24 μg/mL), Fraction-5231 (IC50 = 3.087 μg/mL), and Fraction-52335A (IC50 = 12.93 μg/mL). The crude extract and fractions exhibited high selectivity for E. coli β-GUS against human β-GUS (P < 0.0001, α =0.05). The antimicrobial assay of fractions showed no toxic effects on E. coli. GC-MS profiling of the active fractions identified the compounds present to be similar to essential oil extracts of P. amboinicus reported previously. Ranking of these compounds by in silico identified the compounds with high binding affinity: Phthalic acid, cyclobutyl tridecyl ester (-7.5 kcal/mol) from Fraction-543W, N-Benzyl-2-allyl-2-tosyl-4-penten-1-amine (-8.0 kcal/mol) from Fraction-5231, and Dehydroabietic Acid (-7.9 kcal/mol) from Fraction-52335A. By comparing to binding modes of reported inhibitors, we show that these compounds also interact with active site residues Tyr469 and Tyr472, and with several residues in the β-GUS bacterial loop. Conclusion: Herein, we identified highly-active and highly-selective E. coli β-GUS inhibitors from P. amboinicus leaf chloroform extracts, utilizing a bioassay-guided purification coupled by metabolomics and in silico docking approach. This is the first report on the potential of P. amboinicus as selective inhibitor of E. coli β-GUSs.