中央研究院 生物化學研究所
中央研究院 生物化學研究所
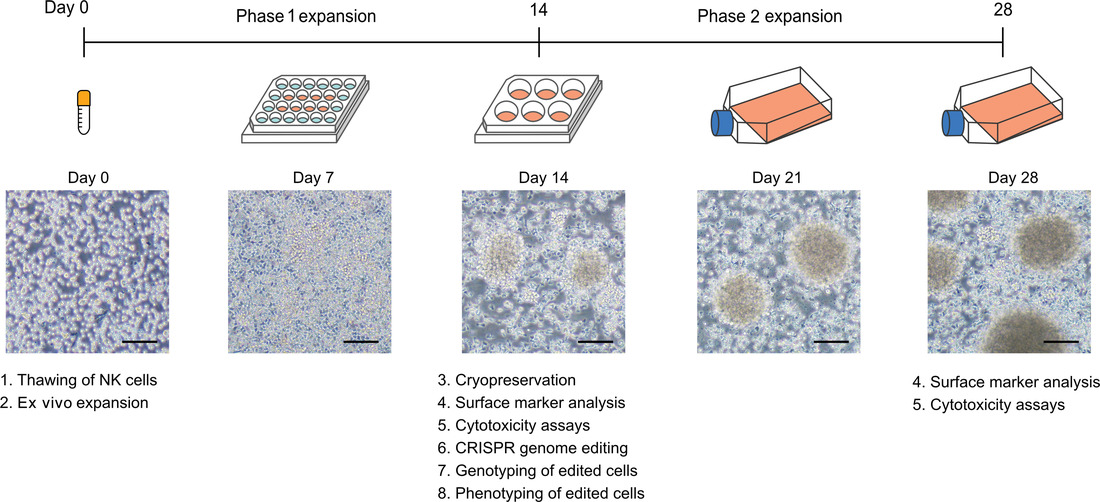
Natural killer (NK) cells are potent innate immune cells that provide the surveillance and elimination of infected, stressed, and malignant cells. The unique immune recognition mechanisms and functions of NK cells make them an attractive cell type for immunology research and adoptive immunotherapy. However, primary NK cells are challenging to culture ex vivo and lack efficient genetic tools, hindering the research of NK cells and the development of NK cell therapeutics. Here we describe methods for the freeze-thaw process, feeder-free ex vivo expansion, CRISPR-Cas9 genome editing, and functional characterizations of primary human NK cells. Our protocol enables ∼30-fold and ∼2000-fold average expansion rates from 1 × 107 cryopreserved NK cells in 14 and 28 days, respectively. We also detail methods for CRISPR gene knockout and knockin by nucleofection of Cas9 ribonucleoproteins (RNP) and DNA repair templates. Gene knockout by Cas9 RNP nucleofection can be multiplexed to simultaneously target three genes. The CRISPR-edited cells can be cryopreserved and rethawed with high viability for future studies. © 2021 Wiley Periodicals LLC.
Basic Protocol 1: Thawing of natural killer cells
Basic Protocol 2: Ex vivo expansion of natural killer cells
Basic Protocol 3: Cryopreservation of expanded natural killer cells
Basic Protocol 4: Characterization of natural killer cells: Flow cytometry and surface marker analysis
Basic Protocol 5: Cytotoxicity and degranulation assays
Basic Protocol 6: Preparation of homology-directed repair templates
Basic Protocol 7: Nucleofection of CRISPR-Cas9 ribonucleoproteins
Basic Protocol 8: Genotyping of gene-edited natural killer cells
Basic Protocol 9: Phenotyping of gene-edited natural killer cells