中央研究院 生物化學研究所
中央研究院 生物化學研究所
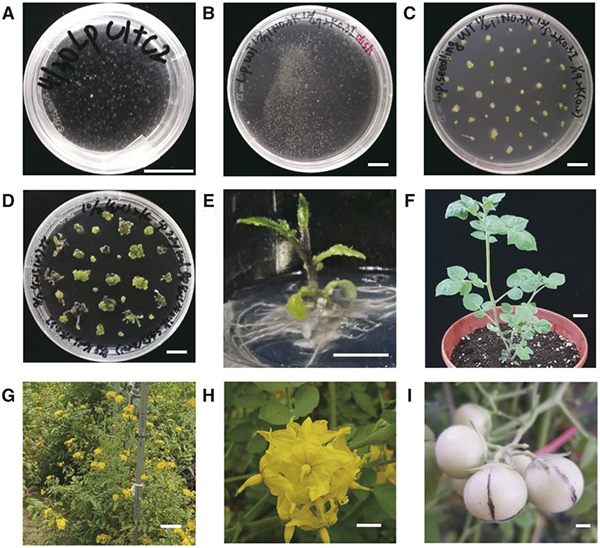
Wild tomatoes (Solanum peruvianum) are important genomic resources for tomato research and breeding. Development of a foreign DNA-free CRISPR-Cas delivery system has potential to mitigate public concern about genetically modified organisms. Here, we established a DNA-free CRISPR-Cas9 genome editing system based on an optimized protoplast regeneration protocol of S. peruvianum, an important resource for tomato introgression breeding. We generated mutants for genes involved in small interfering RNAs (siRNA) biogenesis, RNA-DEPENDENT RNA POLYMERASE 6 (SpRDR6) and SUPPRESSOR OF GENE SILENCING 3 (SpSGS3); pathogen-related peptide precursors, PATHOGENESIS-RELATED PROTEIN-1 (SpPR-1) and PROSYSTEMIN (SpProSys); and fungal resistance (MILDEW RESISTANT LOCUS O, SpMlo1) using diploid or tetraploid protoplasts derived from in vitro-grown shoots. The ploidy level of these regenerants was not affected by PEG-Ca2+-mediated transfection, CRISPR reagents, or the target genes. By karyotyping and whole genome sequencing analysis, we confirmed that CRISPR-Cas9 editing did not introduce chromosomal changes or unintended genome editing sites. All mutated genes in both diploid and tetraploid regenerants were heritable in the next generation. spsgs3 null T0 regenerants and sprdr6 null T1 progeny had wiry, sterile phenotypes in both diploid and tetraploid lines. The sterility of the spsgs3 null mutant was partially rescued, and fruits were obtained by grafting to wild-type stock and pollination with wild-type pollen. The resulting seeds contained the mutated alleles. Tomato yellow leaf curl virus proliferated at higher levels in spsgs3 and sprdr6 mutants than in the wild type. Therefore, this protoplast regeneration technique should greatly facilitate tomato polyploidization and enable the use of CRISPR-Cas for S. peruvianum domestication and tomato breeding.