中央研究院 生物化學研究所
中央研究院 生物化學研究所
中央研究院 生物化學研究所

 中央研究院 生物化學研究所
中央研究院 生物化學研究所
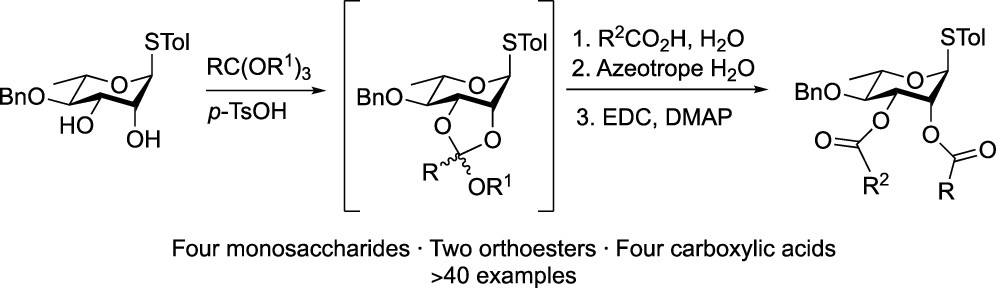
A one-pot strategy for functionalizing pyranoside 1,2-cis-diols with two different ester protecting groups is reported. The approach employs regioselective acylation via orthoester hydrolysis promoted by a carboxylic acid, e.g., levulinic acid, acetic acid, benzoic acid, or chloroacetic acid. Upon removal of water and introduction of a coupling agent, the carboxylic acid is esterified to the hydroxyl group liberated during hydrolysis. Although applied to 1,2-cis-diols on pyranoside scaffolds, the method should be applicable to such motifs on any six-membered ring.