中央研究院 生物化學研究所
中央研究院 生物化學研究所
中央研究院 生物化學研究所

 中央研究院 生物化學研究所
中央研究院 生物化學研究所
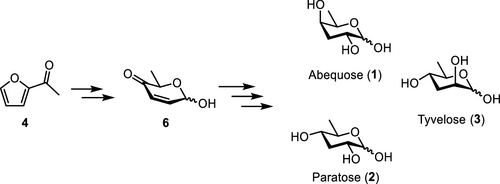
We report the de novo asymmetric synthesis of the 3,6-dideoxy sugars abequose, paratose, and tyvelose from 2-acetylfuran. Conversion of this readily available ketone to a pyranone derivative was followed by transformation to either an α- or β-glycoside via diasteroselective acylation. Michael addition at C2 controlled primarily by the C1 configuration in the glycoside produced 3,6-dideoxy-4-keto sugars, which could be reduced and converted to either fully deprotected monosaccharides or to immediate precursors of glycosyl donors.