中央研究院 生物化學研究所
中央研究院 生物化學研究所
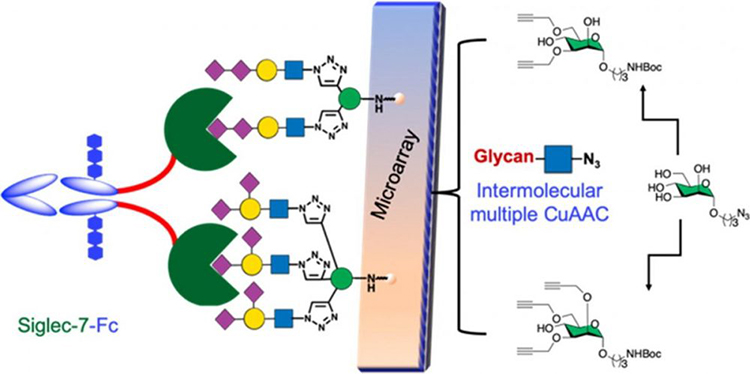
Naturally occurring N-glycans display much diversity in modifications, linkages, and peripheral presentation of the oligosaccharide chain. Despite continued advancements in oligosaccharide synthesis, synthetic access to these natural glycans remains challenging. Biologically relevant complex N-glycan mimetics with various natural and unnatural modifications are an alternate way for investigating glycan-protein interactions. Further supporting this pattern, we report here a new class of sialylated bi- and triantennary pseudo mannose N-glycans reproducing orientation of the underlying glycan chain and branching patterns and replacing the two inner mannopyranosyl units with 1,2,3-triazole rings. Such mimetics are straightforwardly generated by implementing multiple intermolecular Cu(I)-catalyzed azide-alkyne cycloaddition between chemoenzymatically synthesized azido sialosides and rationally designed C-3 and C-6 di-O- or C-2, C-3, and C-6 tri-O-alkynylated mannoside. Human recombinant Siglec-7-Fc fusion protein recognizes almost all sialylated pseudo mannose N-glycans in the microarray. However, a differential Sia-binding pattern was also observed. Given the library size, comparison of pairwise mannose N-glycan combinations showed that biantennary linear α(2,3)α(2,8)- and α(2,6)α(2,8)- or branched α(2,3)α(2,6)-, and triantennary branched α(2,3)α(2,6)-sialyl pseudo N-glycans possess similar binding capabilities and affinity to recombinant Siglec-7-Fc. While the full range of topological mannose arms remain elusive, the bi- and triantennary mimics are simpler structures for interrogating Siglec interactions.