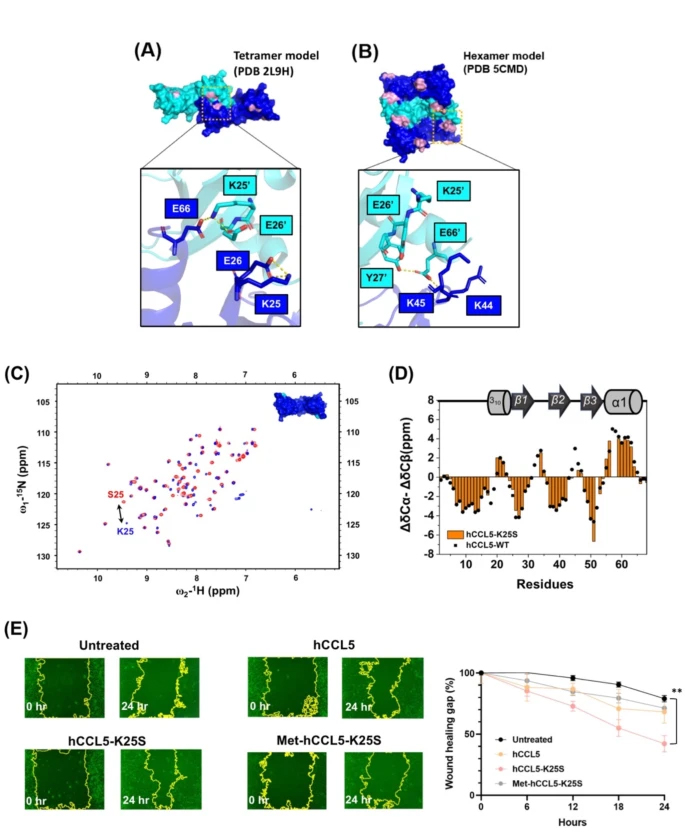
Human inflammation-related CC chemokine ligand 5 (hCCL5) has significant self-assembly property under physiological conditions. The mechanism and function of hCCL5 oligomerization remain unclear. Different intermolecular interactions, such as E66-K25 or E66-R44/K45, have been reported to mediate hCCL5 oligomerization. This complexity makes structural determination difficult. Based on a K25S mutation to eliminate the E66-K25 interaction, we observed hCCL5 forming a helical-sharped filament in transmission electron microscopy (TEM). The filamentous polymerization is a dominant process when the concentration reaches ~ 100 nM and the filaments further form a higher-order assembly when concentration increases. In this large filament, a combination of X-ray solution scattering and cryo-EM analysis determined the structure; NMR further confirmed the filament packing, in which the interactions of residues R44 and K45 are critically involved. The sequence 43TRKNR47 was found to be essential for CCL5 trafficking inside the cells and for glycosaminoglycan binding outside the cells. The functional aspects of the chemokine filament are discussed.

 Institute of Biological Chemistry, Academia Sinica
Institute of Biological Chemistry, Academia Sinica