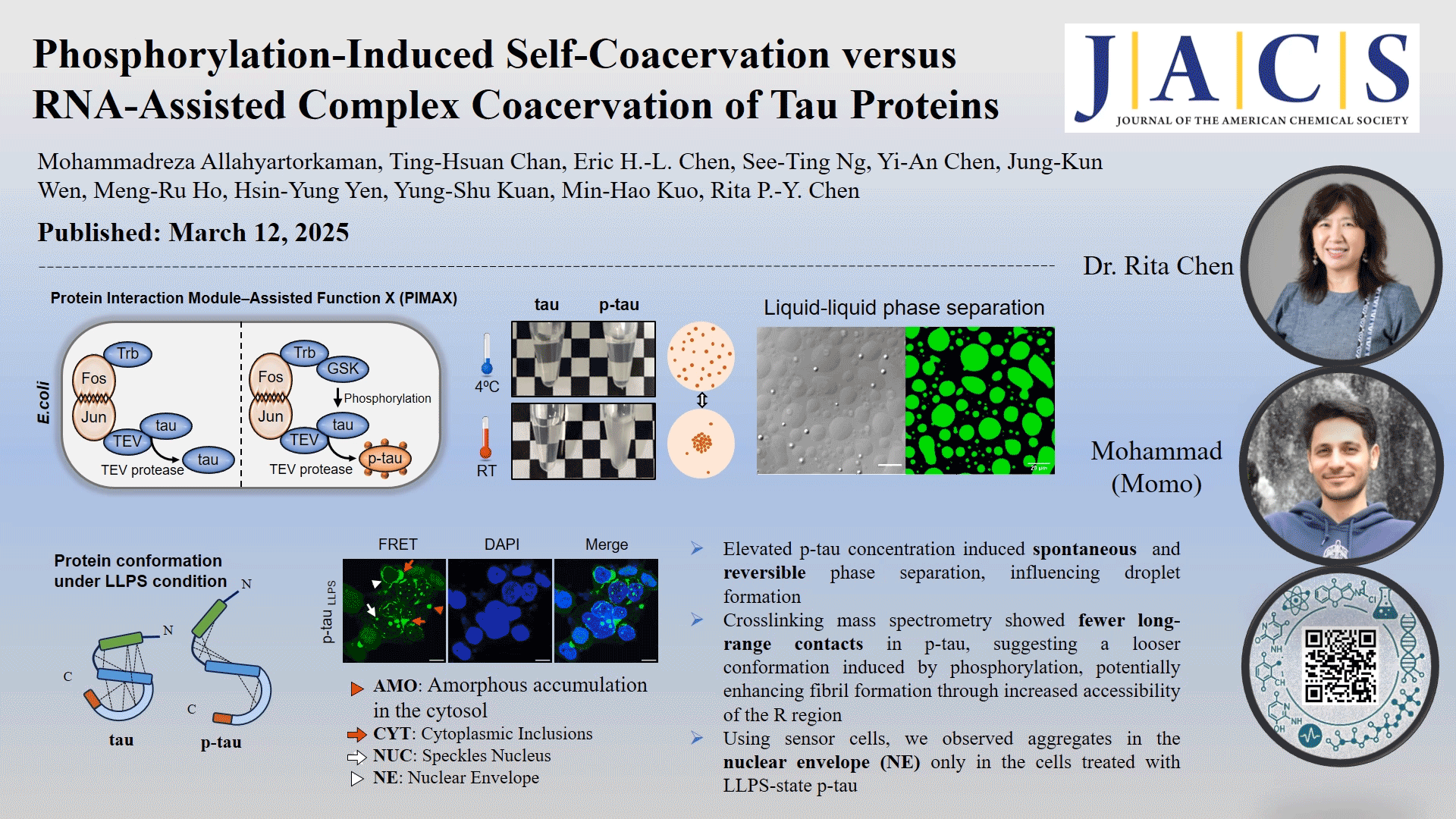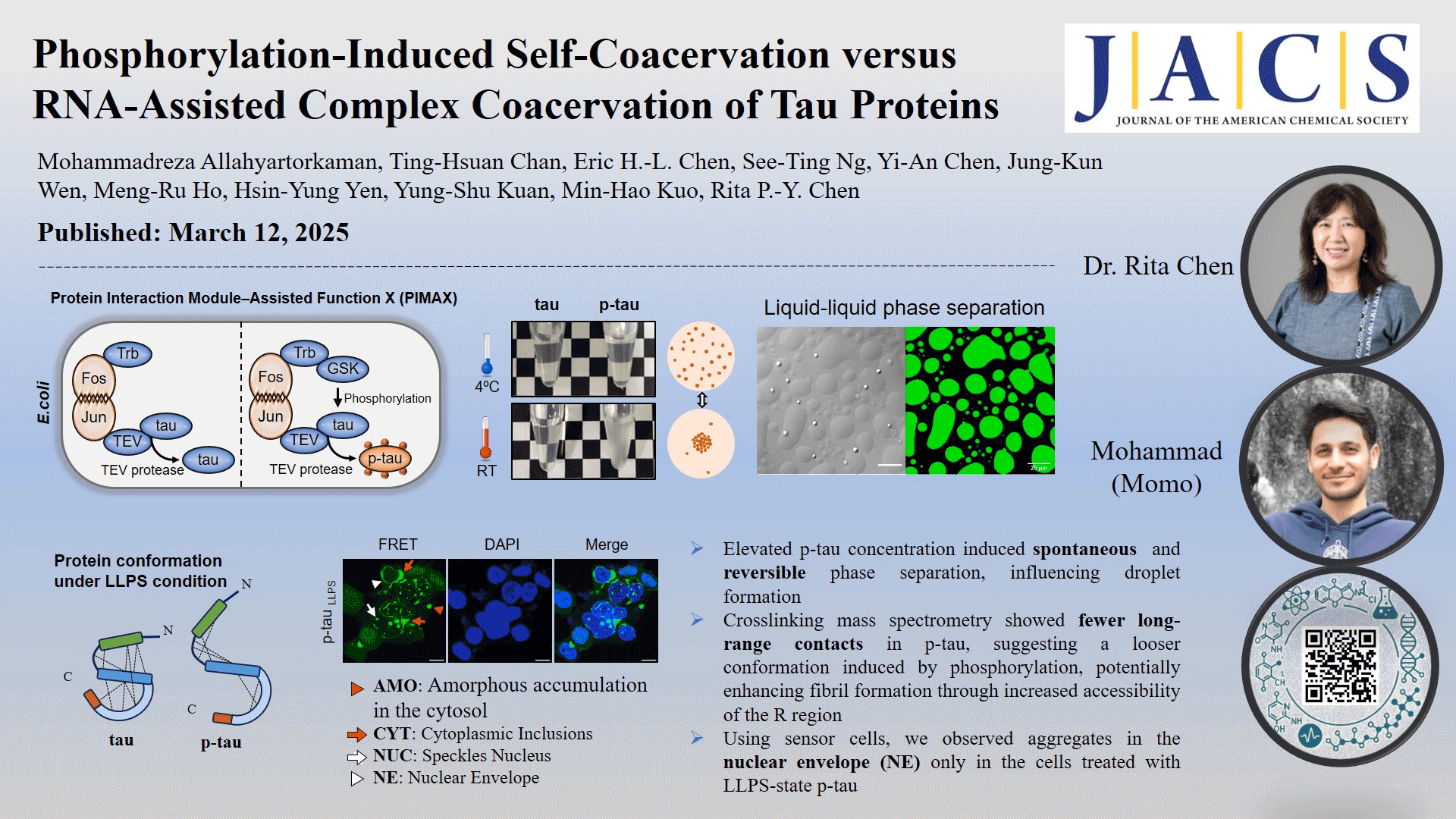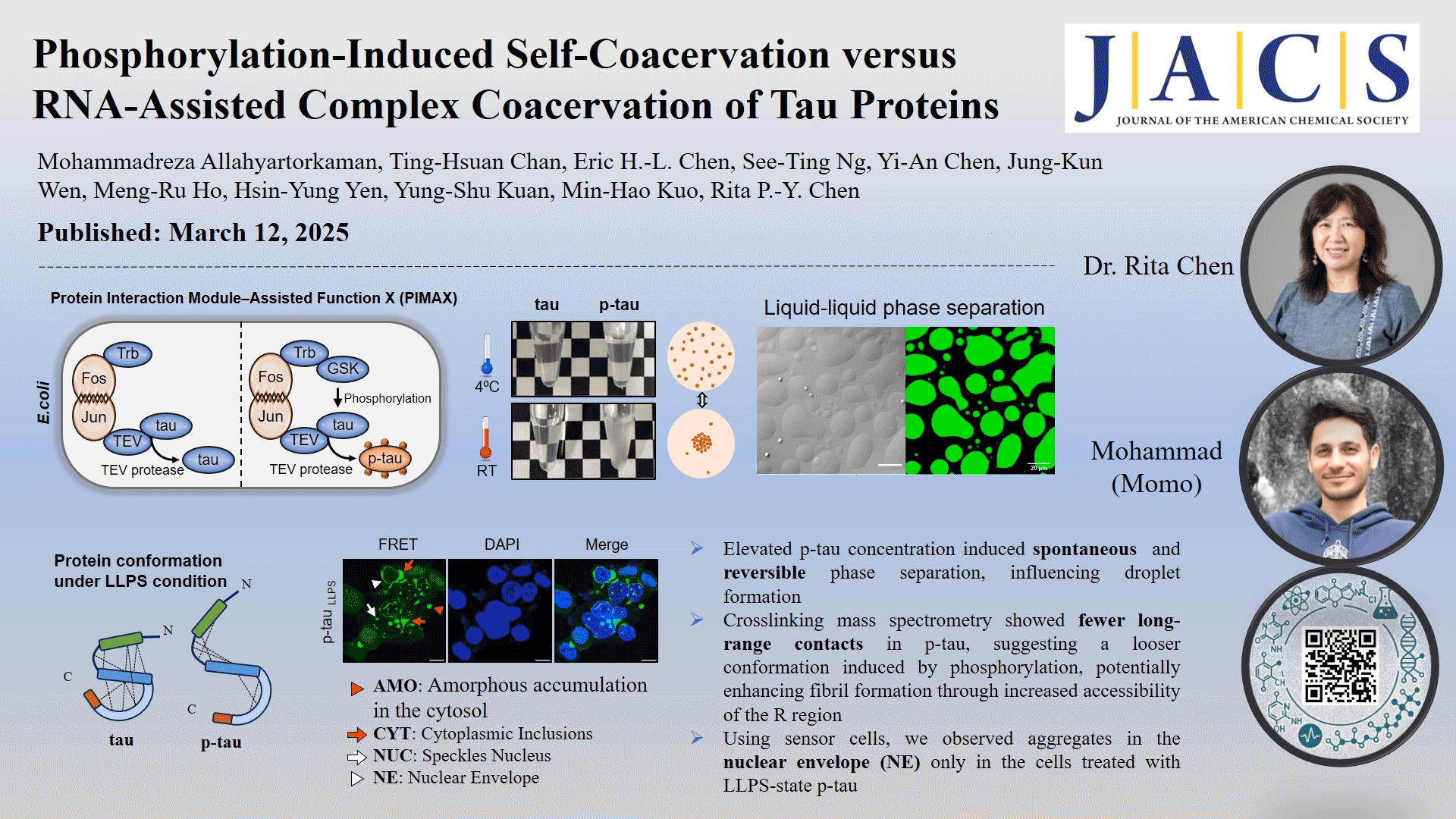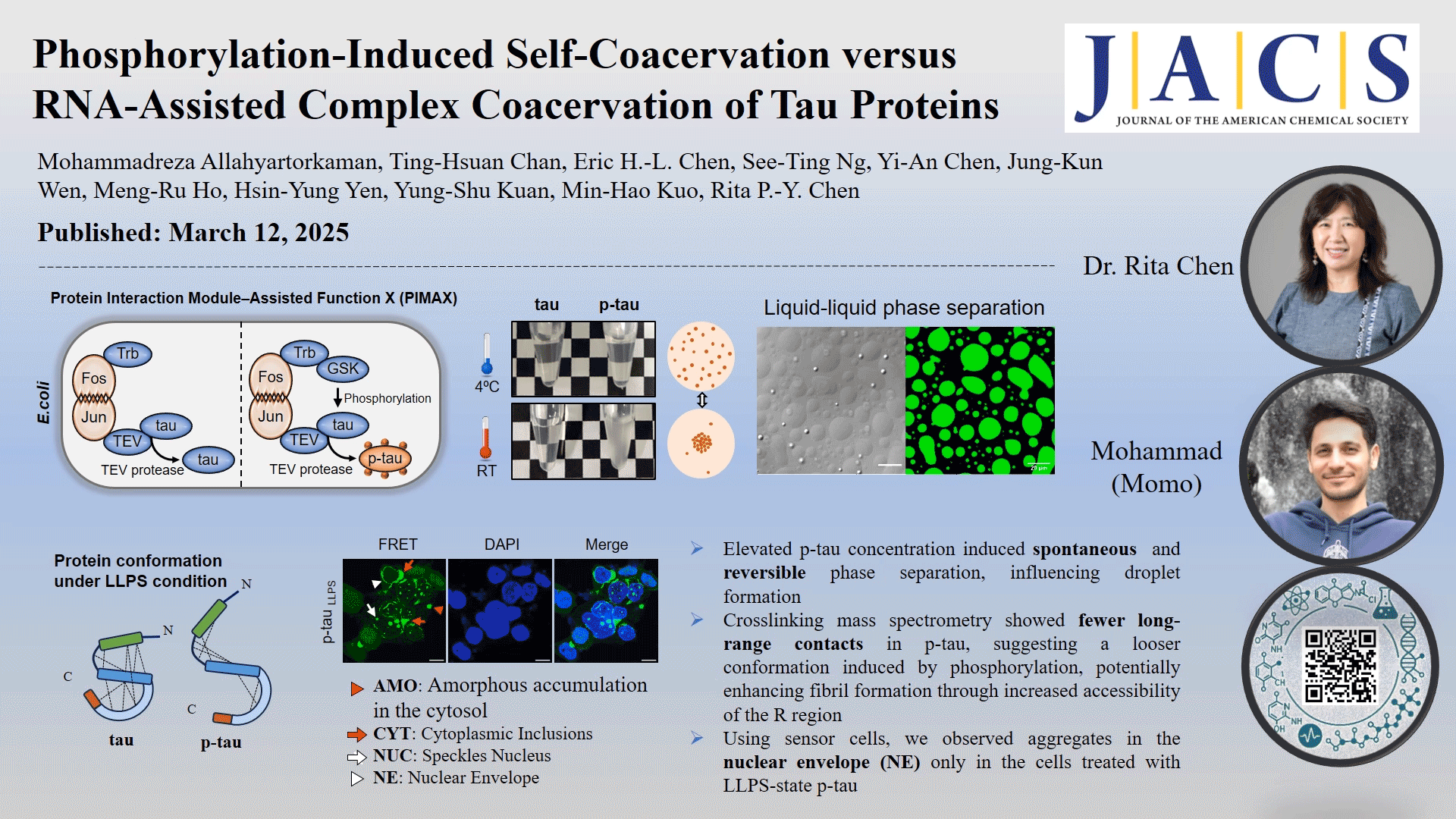
In this study, the role of phosphorylation in the liquid-liquid phase separation (LLPS) of tau, the underlying driving forces, and the potential implications of this separation on protein conformation and subsequent protein aggregation were investigated. We compared in vivo-produced phosphorylated tau (p-tau) and nonphosphorylated tau under different coacervation conditions without adding crowding agents. Our findings revealed that spontaneous phase separation occurs exclusively in p-tau, triggered by a temperature shift from 4 °C to room temperature, and is driven by electrostatic and hydrophobic interactions. The p-tau self-acervation is reversible with temperature changes. Native mass spectrometry detects only two to nine phosphate groups per p-tau molecule, highlighting the impact of phosphorylation on tau's structural flexibility. Cross-linking mass spectrometry showed fewer long-range contacts in p-tau, suggesting a looser conformation induced by phosphorylation. Phosphorylation-induced LLPS and RNA-induced LLPS occurred at different timeframes. However, neither tau nor p-tau formed fibrils without the addition of dextran sulfate or RNA as inducers. Using human kidney epithelial cells expressing the tau R domain fused with fluorescent proteins as reporter cells, we observed aggregates in the nuclear envelope (NE) only in the cells treated with LLPS-state p-tau, which correlates with NE occurrences reported in Alzheimer's disease brain sections. These findings provide deeper insights into the impact of phosphorylation on tau aggregation through an intermediate condensation phase, offering novel perspectives on neurodegenerative disease mechanisms.

 Institute of Biological Chemistry, Academia Sinica
Institute of Biological Chemistry, Academia Sinica