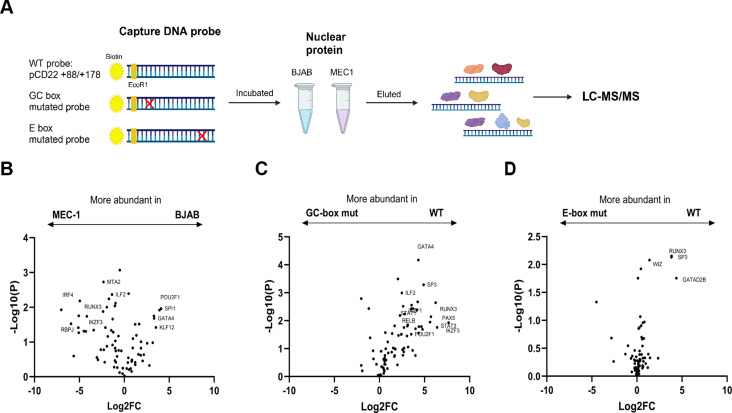
CD22 (also known as Siglec-2) is a member of the Siglec family of glycan-recognition proteins and functions as a negative regulator of the B-cell receptor-mediated calcium signaling. Although the low level of CD22 expression on the B cells in patients with chronic lymphocytic leukemia (CLL) has been documented, CD22's role and its down-regulation mechanism in CLL are yet to be fully studied. In this study, we confirmed that the surface CD22 protein and its mRNA are down-regulated in the B cells of CLL patients. We analyzed a public transcriptomic dataset and found that the CD22 mRNA level is negatively associated with the prognosis of patients with CLL. To investigate the mechanism of CD22 down-regulation, we characterized the minimal promoter of the human CD22 gene required for its transcriptional activation in B cell lines. We employed an unbiased proteomic approach to identify several transcription factors binding to the minimal CD22 promoter, including PU.1, Spi-B, and IRF4. The chromatin immunoprecipitation-quantitative PCR revealed that PU.1 was enriched in a CD22-high cell line, while IRF4 was enriched in a CD22-low cell line. We then conducted over-expression/knockout/knockdown experiments, which validated that PU.1 and Spi-B positively, and IRF4 negatively, regulate CD22 transcription. Our study thus provides insights into the transcriptional regulation of CD22 and the mechanism by which CD22 expression is down-regulated in the B cells of patients with CLL.

 Institute of Biological Chemistry, Academia Sinica
Institute of Biological Chemistry, Academia Sinica