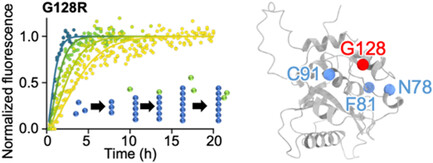
BRCA1-associated protein-1 (BAP1) is a tumor suppressor protein that regulates DNA transcription through its deubiquitinase activity. Cancer-associated missense mutations within its ubiquitin C-terminal hydrolase (UCH) domain result in structural destabilization and aggregation, contributing to various malignancies such as malignant mesothelioma and uveal melanoma. In this study, we investigated the aggregation mechanisms of highly destabilized BAP1-UCH variants, including N78S, C91W, F81V, and G128R, using Thioflavin T (ThT) binding assays and AmyloFit analysis. Our results reveal that all BAP1-UCH variants follow a secondary nucleation-dominated aggregation model, exhibiting strong concentration dependence and significantly higher aggregation rates, which may be responsible for their impaired nuclear import, leading to increased cytosolic retention. These findings provide critical insights into how specific mutations in BAP1 drive aggregation and compromise its tumor-suppressing functions inside the nucleus, thereby contributing to cancer progression.

 Institute of Biological Chemistry, Academia Sinica
Institute of Biological Chemistry, Academia Sinica