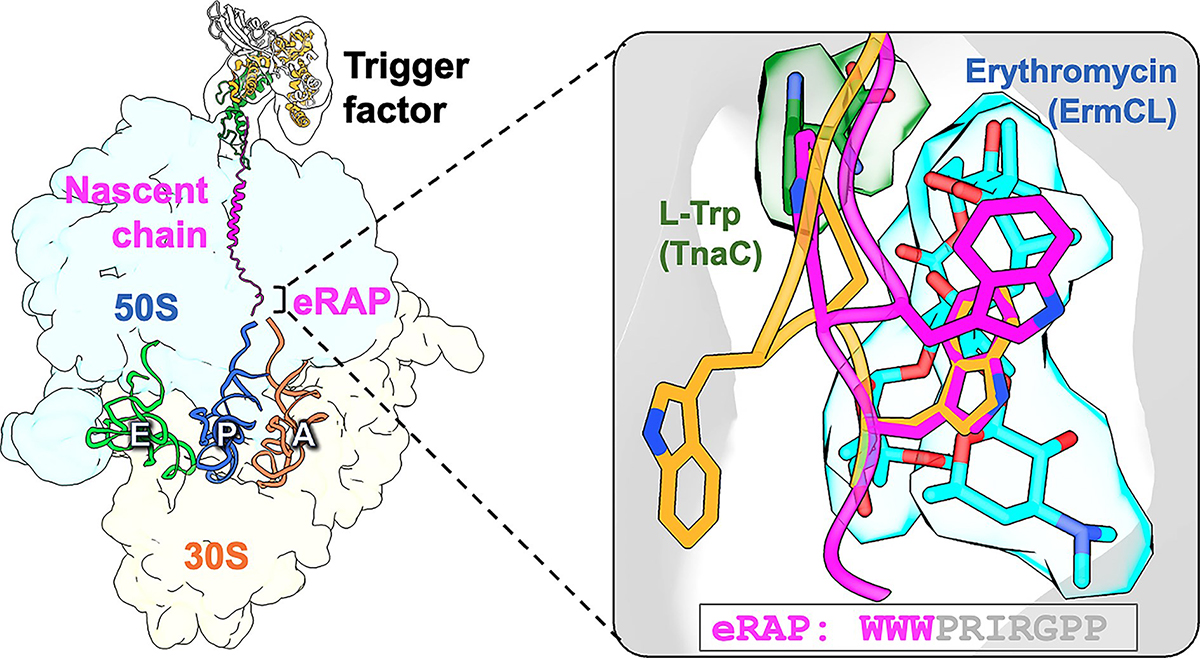
Ribosome stalling is a critical regulatory mechanism in protein synthesis, controlling the rate and fidelity of translation. Arrest peptides, short sequences within nascent chains, can induce ribosome stalling, providing insights into the dynamics of translation and potential therapeutic targets. In this study, we investigated the molecular mechanisms of ribosome stalling induced by an engineered ribosomal arrest peptide (eRAP). We used cryo-electron microscopy and biochemical assays to characterize the interactions between eRAP, the ribosome, and accessory factors such as the trigger factor. Our results reveal intricate details of the eRAP-induced ribosome stalling, including the conformational changes in the ribosomal tunnel and the nascent chain. We also observed interactions between eRAP and specific ribosomal components, highlighting the role of key amino acids in mediating ribosome stalling. Furthermore, comparison with other stalling mechanisms, such as those induced by antibiotics or natural nascent peptides, elucidates the unique features of eRAP-induced stalling. Overall, our findings provide a comprehensive understanding of ribosome stalling by arrest peptides, shedding light on the fundamental processes of translation and offering potential avenues for therapeutic interventions targeting translation regulation.

 Institute of Biological Chemistry, Academia Sinica
Institute of Biological Chemistry, Academia Sinica