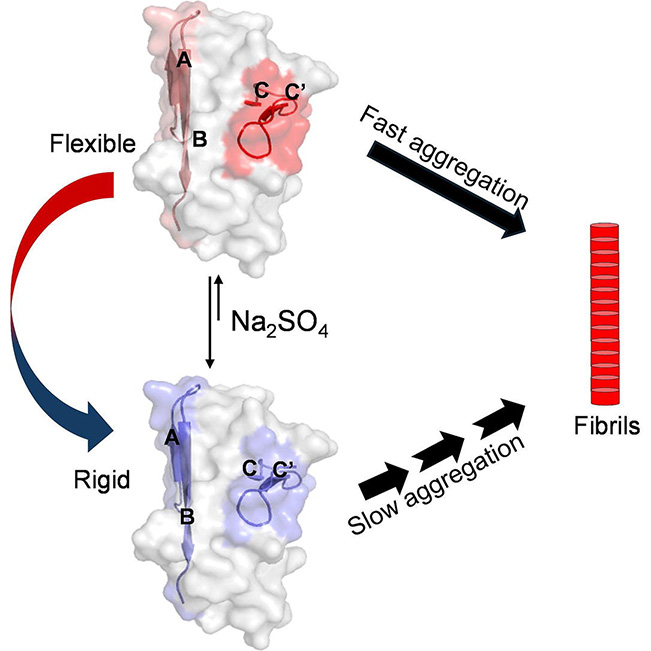
Light chain (AL) amyloidosis is a life-threatening systemic disorder caused by the aggregation and deposition of antibody light chain (LC) fragments in multiple organs, including the heart and kidneys. In this study, we investigated the early events of aggregation of the highly unstable variable domain (VL) from AL55, a known amyloidogenic and cardiotoxic light chain. Our results show that dimer disruption and exposure of aggregation hot spots synergistically accelerate aggregation. At neutral pH, concentration-dependent dimerization reduces aggregation by limiting aggregation-competent monomers. Dilution or lowering the pH disrupts dimerization, exposes aggregation-prone regions (APRs), and accelerates aggregation. In contrast, when APRs are chemically stabilized, the aggregation rate decreases despite high monomer availability. Together, this study establishes that AL55 VL domain aggregation is regulated by dimer dissociation, electrostatic modulation, and formation of an aggregation-competent conformation involving a dynamic N-terminal (residues 5-26) and dimeric interface (residues 38-56) region, ultimately yielding structurally compact and highly stable fibrils.

 Institute of Biological Chemistry, Academia Sinica
Institute of Biological Chemistry, Academia Sinica